Types of Chemical Bonds Worksheet
Chemical bonding is a fundamental concept in chemistry, and understanding the different types of bonds is crucial for students studying this subject. A chemical bonds worksheet can be a valuable tool for reinforcing knowledge and improving understanding. In this blog post, we will discuss the various types of chemical bonds and how a worksheet can aid students in mastering this topic. Whether you're a high school student preparing for exams or a college student looking to deepen your understanding, this worksheet can provide a comprehensive review of chemical bonds.
Table of Images 👆
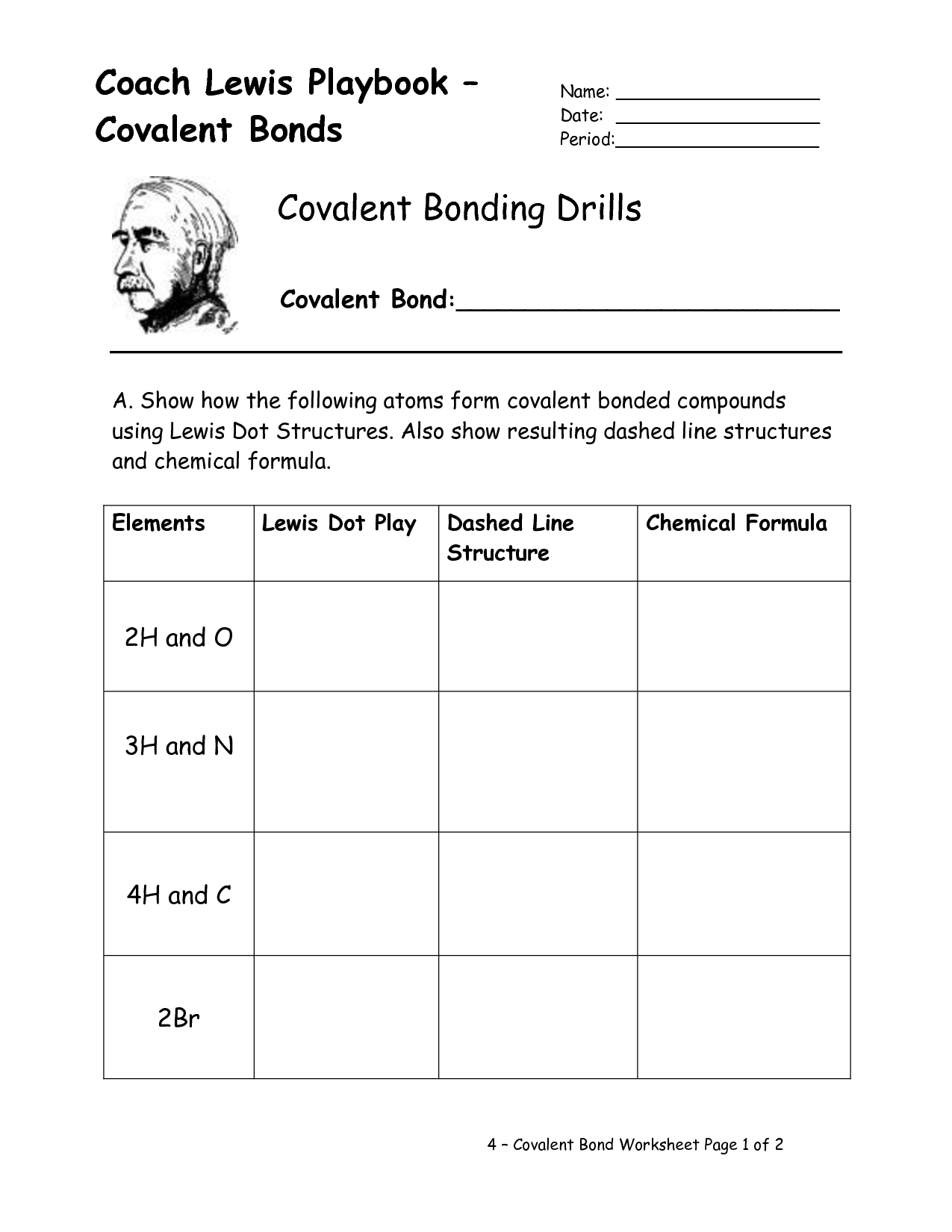
- Lewis Dot Covalent Bond Worksheet
- Practice Ionic Covalent Compound Worksheet
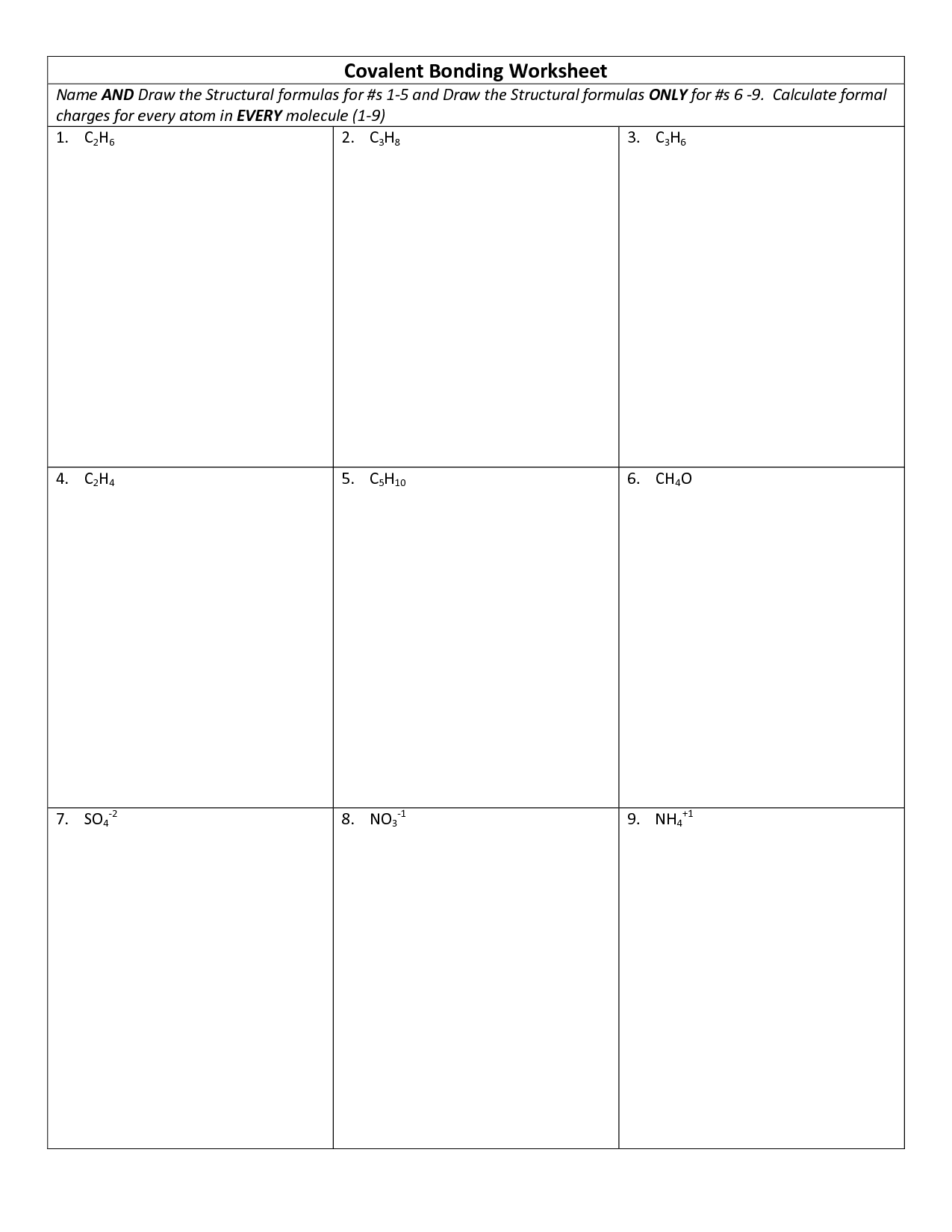
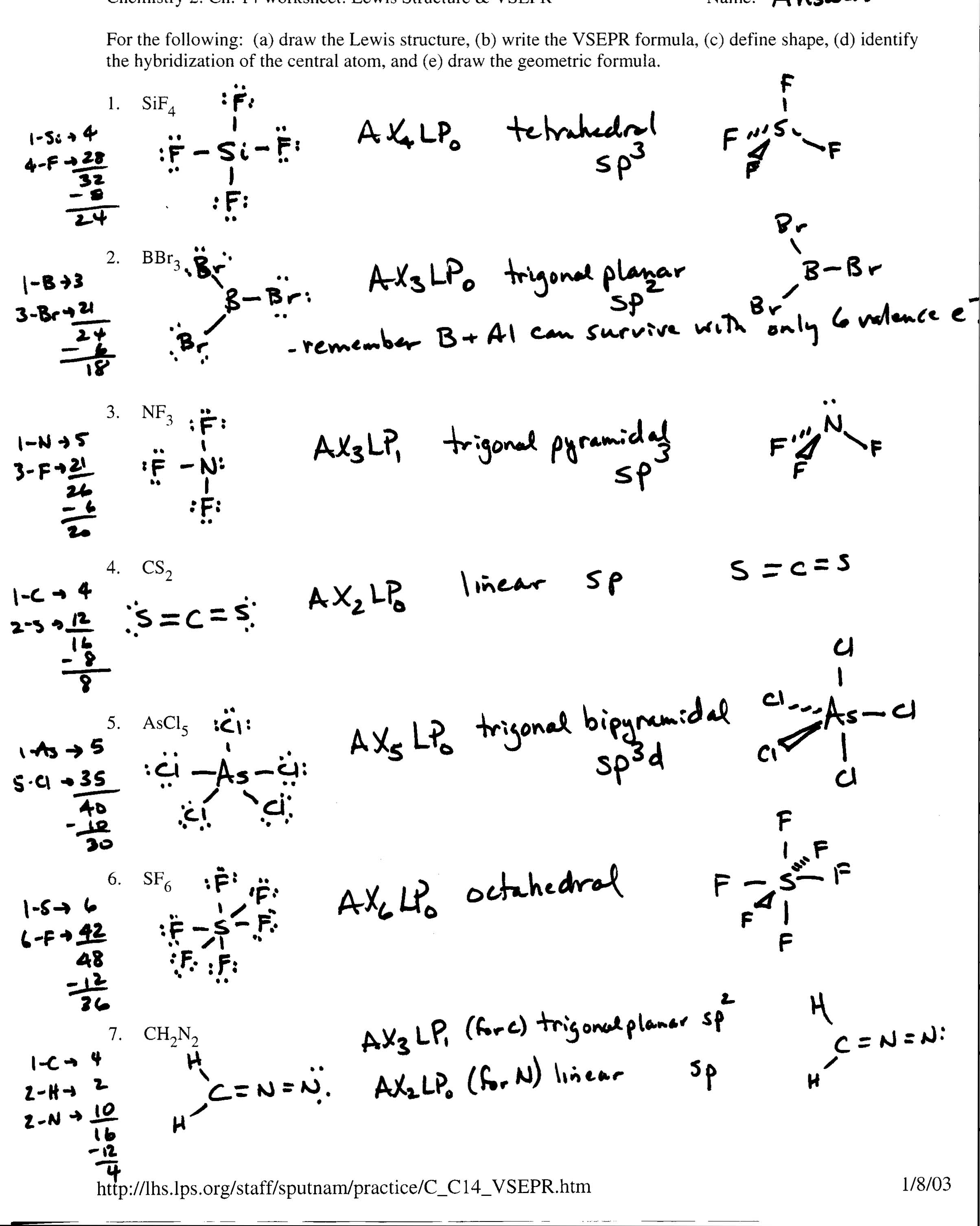
- Covalent Bonding Lewis Structures Worksheet
- Balancing Chemical Equations Worksheet Answer Key
- Chemical Bonding Worksheet Answers
- Chemistry Worksheet Answer Keys
- Chapter 8 Covalent Bonding Worksheet Answer Key
- Covalent Bonding Worksheet Answers
- Organic Chemistry Worksheets Answers
- Chemistry of Life Answer Key Chapter 2
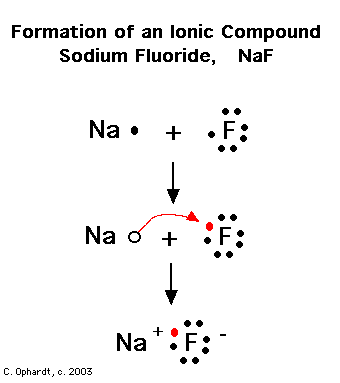
- NAF Lewis Dot Structure
- Molar Mass Practice Worksheet
- Molar Mass Practice Worksheet
- Molar Mass Practice Worksheet
- Molar Mass Practice Worksheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
What is an ionic bond?
An ionic bond is a type of chemical bond that forms between two atoms when one atom transfers one or more electrons to another atom. This results in the production of positively charged ions (cations) and negatively charged ions (anions), which are attracted to each other due to their opposite charges, creating a strong electrostatic force that holds the atoms together in a bond.
What is a covalent bond?
A covalent bond is a type of chemical bond where two atoms share a pair of electrons in order to achieve a more stable electron configuration. This sharing of electrons results in the formation of molecules and compounds, with the atoms being held together by the electrostatic forces between the shared electrons and the positively charged nuclei of the atoms involved. Covalent bonds are typically found in non-metallic elements and compounds.
What is a metallic bond?
A metallic bond is a type of chemical bonding that occurs between metal atoms, where the electrons are shared and free to move between the atoms. This results in the formation of a lattice structure where metal ions are held together by a "sea" of delocalized electrons, which contributes to the high electrical and thermal conductivity, malleability, and ductility of metals.
What is a hydrogen bond?
A hydrogen bond is a strong electrostatic attraction between a hydrogen atom bonded to an electronegative element (such as oxygen, nitrogen, or fluorine) and a neighboring electronegative atom. It is a type of intermolecular force that is weaker than covalent or ionic bonds but plays a crucial role in determining the structure and properties of many compounds, such as water and biological molecules like DNA and proteins.
What is a polar covalent bond?
A polar covalent bond is a type of chemical bond where two atoms share electrons unevenly, resulting in one atom being slightly more negative and the other slightly more positive. This unequal sharing of electrons is due to differences in electronegativity between the atoms involved, causing a partial separation of charges within the molecule.
What is a nonpolar covalent bond?
A nonpolar covalent bond is a type of chemical bond where two atoms share electrons equally due to similar electronegativities, meaning the electrons are not pulled closer to one atom than the other. This results in a symmetrical distribution of charge and no distinct separation of charge within the molecule, making it nonpolar.
What is a dative covalent bond?
A dative covalent bond, also known as a coordinate covalent bond, is a type of covalent bond where both of the shared electrons come from one of the atoms involved in the bond. This differs from a typical covalent bond where each atom contributes one electron to the shared pair. In a dative covalent bond, one atom donates both electrons to form the bond while the other atom does not contribute any electrons.
What is an intermolecular bond?
An intermolecular bond refers to the forces of attraction between molecules that hold them together in a condensed phase such as a solid or liquid. These bonds are weaker than chemical bonds within molecules, such as covalent or ionic bonds, and include interactions like hydrogen bonding, van der Waals forces, and dipole-dipole interactions. These intermolecular bonds play a crucial role in determining the physical properties of substances like melting and boiling points, solubility, and viscosity.
What is an intramolecular bond?
An intramolecular bond refers to a chemical bond that occurs within a single molecule, holding together the atoms within that molecule. Examples of intramolecular bonds include covalent bonds, ionic bonds, and metallic bonds that form between the different atoms in a molecule to create a stable structure. By contrast, intermolecular bonds are the forces of attraction that exist between separate molecules.
What is a coordinate covalent bond?
A coordinate covalent bond, also known as a dative bond, is a type of covalent bond in which both electrons come from the same atom. One atom provides both electrons to form the bond, while the other atom does not contribute any electrons. This results in a shared electron pair, where one atom donates a pair of electrons to another atom, forming a stable bond between the two atoms.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.





























Comments