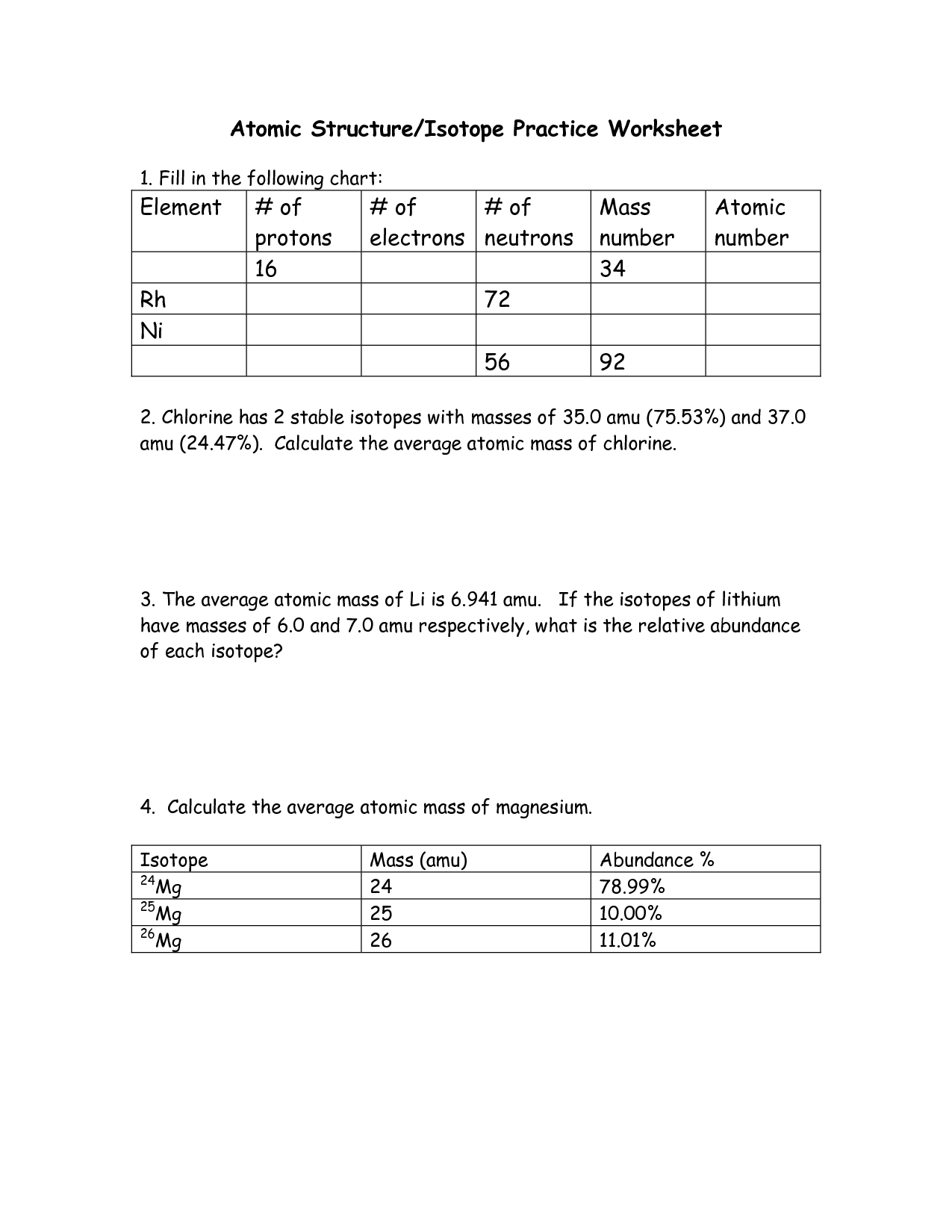
Protons Neutrons and Electrons Practice Worksheet
If you're a student or teacher looking for a useful resource to reinforce your understanding of protons, neutrons, and electrons, then this protons, neutrons, and electrons practice worksheet is exactly what you need. This worksheet provides a comprehensive set of questions and exercises that will help you solidify your knowledge of these fundamental particles of matter.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is the charge of a proton?
The charge of a proton is positive and equal to +1 elementary charge, which is approximately 1.602 x 10^-19 coulombs.
What is the charge of a neutron?
A neutron has no charge.
What is the charge of an electron?
The charge of an electron is negative, specifically -1 elementary charge.
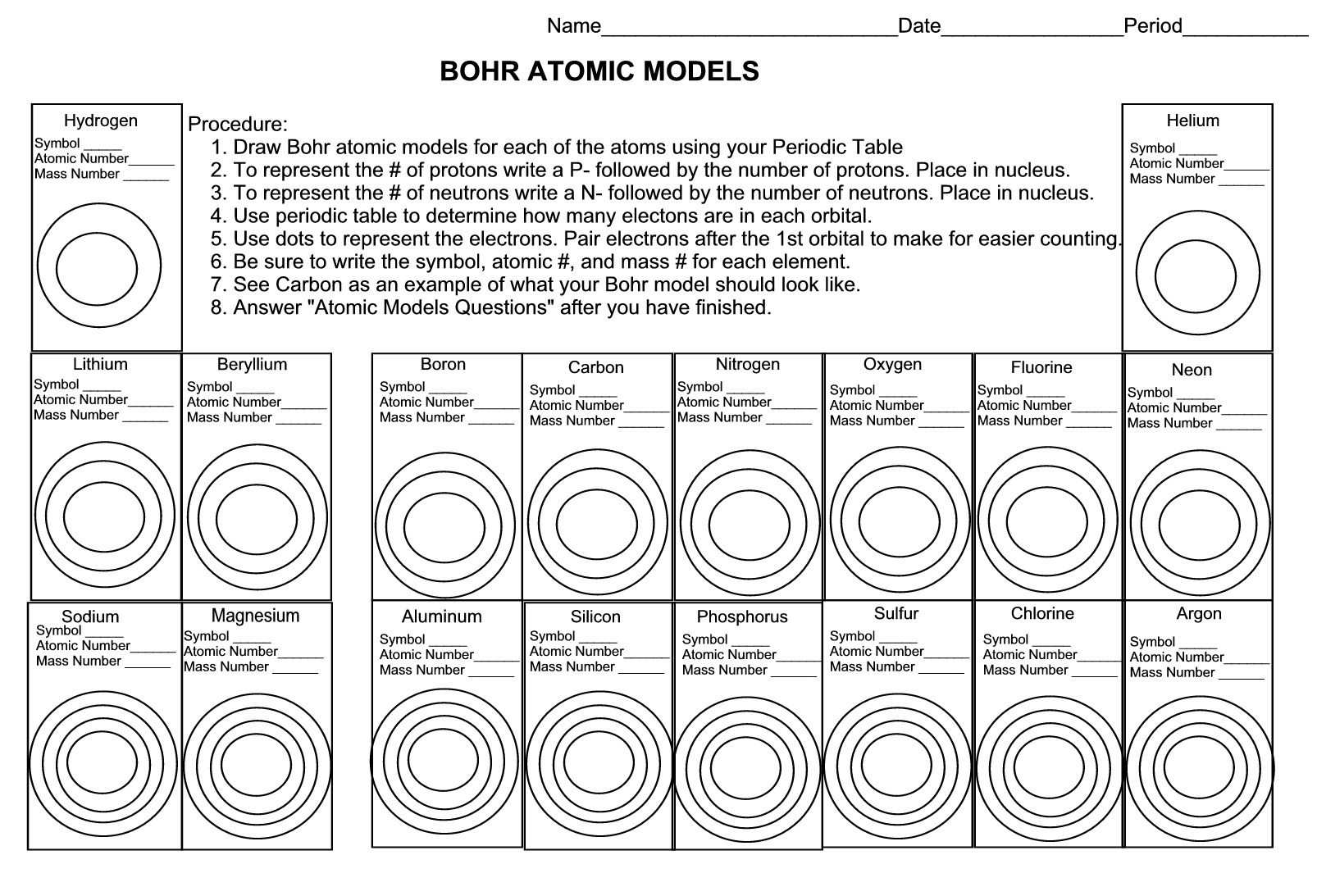
Where are protons located in an atom?
Protons are located in the nucleus of an atom.
Where are neutrons located in an atom?
Neutrons are located in the nucleus of an atom, along with protons. The nucleus is the central core of an atom where most of its mass is concentrated, and it contains positively charged protons and neutral neutrons bound together by strong nuclear forces.
Where are electrons located in an atom?
Electrons are located in specific energy levels or orbitals surrounding the nucleus of an atom. These negatively charged particles are constantly in motion, buzzing around the nucleus in a cloud-like structure that represents the probability of finding an electron in a particular region of space.
What is the relative mass of a proton?
The relative mass of a proton is approximately 1 atomic mass unit (amu).
What is the relative mass of a neutron?
The relative mass of a neutron is approximately 1 atomic mass unit (amu) or 1.00866491588 atomic mass units to be precise. This mass is slightly greater than that of a proton, which is approximately 1.007276 amu.
What is the relative mass of an electron?
The relative mass of an electron is approximately 0.0005 atomic mass units (amu).
What is the significance of protons, neutrons, and electrons in determining the chemical behavior of an atom?
Protons, neutrons, and electrons are the fundamental particles that make up an atom. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge. The number of protons determines the element an atom belongs to, while the number of electrons determines its chemical properties. Neutrons help stabilize the nucleus of the atom. The arrangement of electrons around the nucleus, specifically in the outermost energy level, dictates how atoms interact with other atoms, forming bonds and determining chemical behavior such as reactivity, stability, and bonding characteristics.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.





















Comments