Phase Changes of Matter Worksheet
Are you teaching a science lesson on the phase changes of matter? If so, you'll need a worksheet that effectively explains and assesses your students' understanding of this topic. Look no further! This blog post will introduce you to a fantastic entity that offers a comprehensive and subject-specific worksheet on phase changes of matter. With this worksheet, you can ensure that your students grasp the concept of how substances transition between solid, liquid, and gas phases.
Table of Images 👆
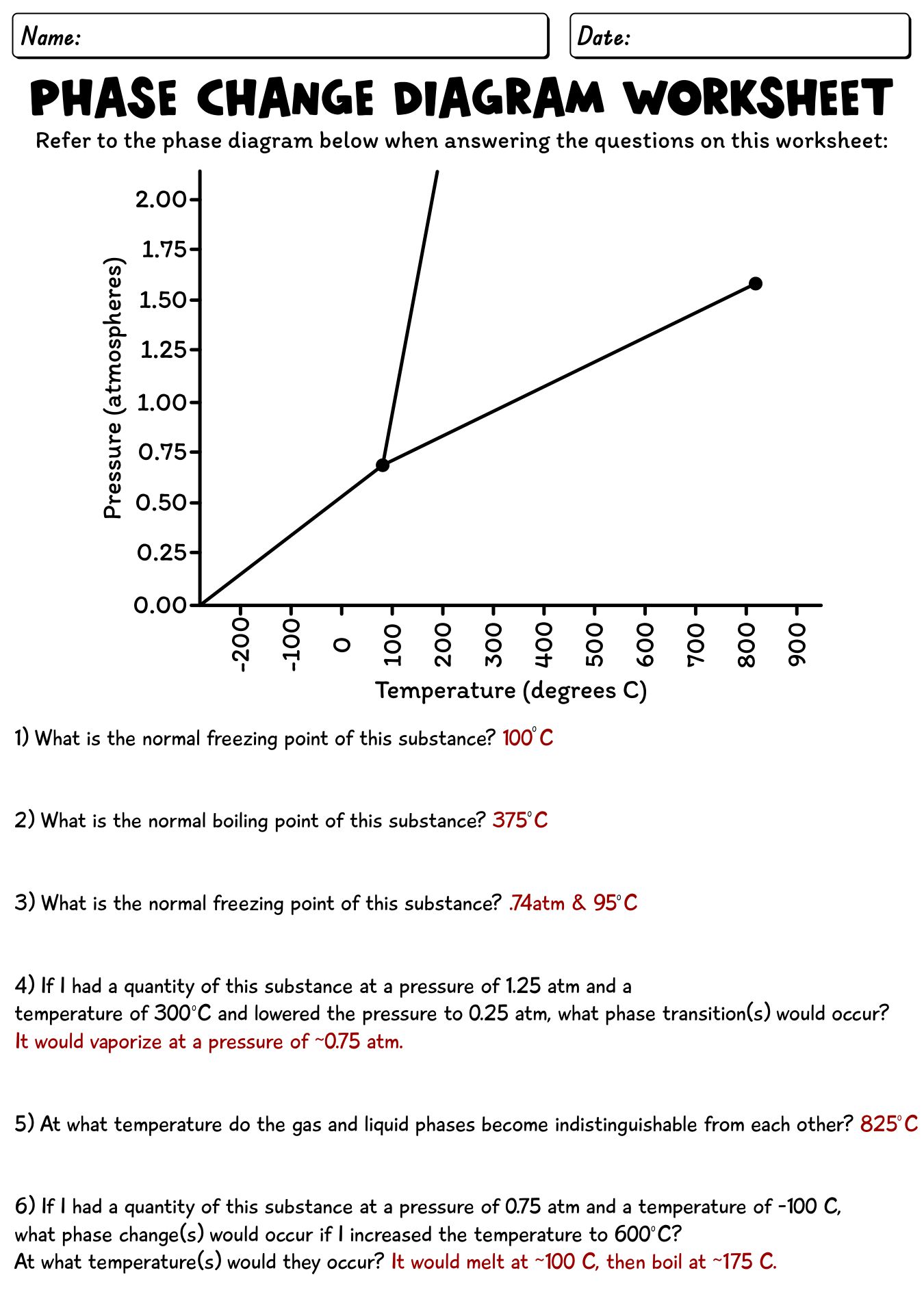
- Phase Change Worksheet Answer Key
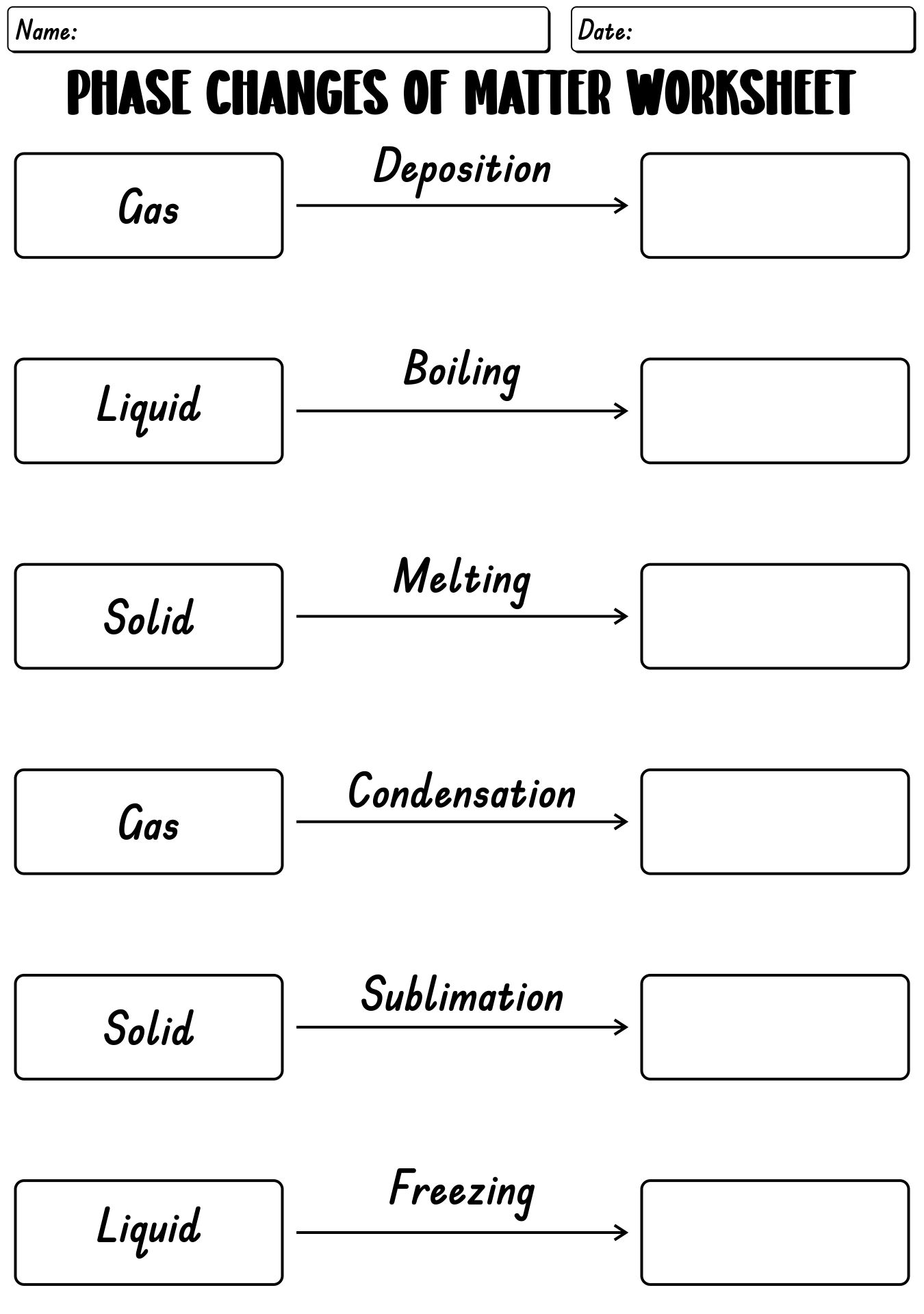
- States of Matter Phase Changes Worksheet
- States of Matter Phase Changes Worksheet
- Properties of Matter Worksheet Answers
- Phase Change Worksheet Answer Key
- Phase Change Worksheet Answer Sheet
- Physical vs Chemical Change Worksheet
- Phase Change Worksheet Answers
- Heat and Phase Changes Worksheet Answers
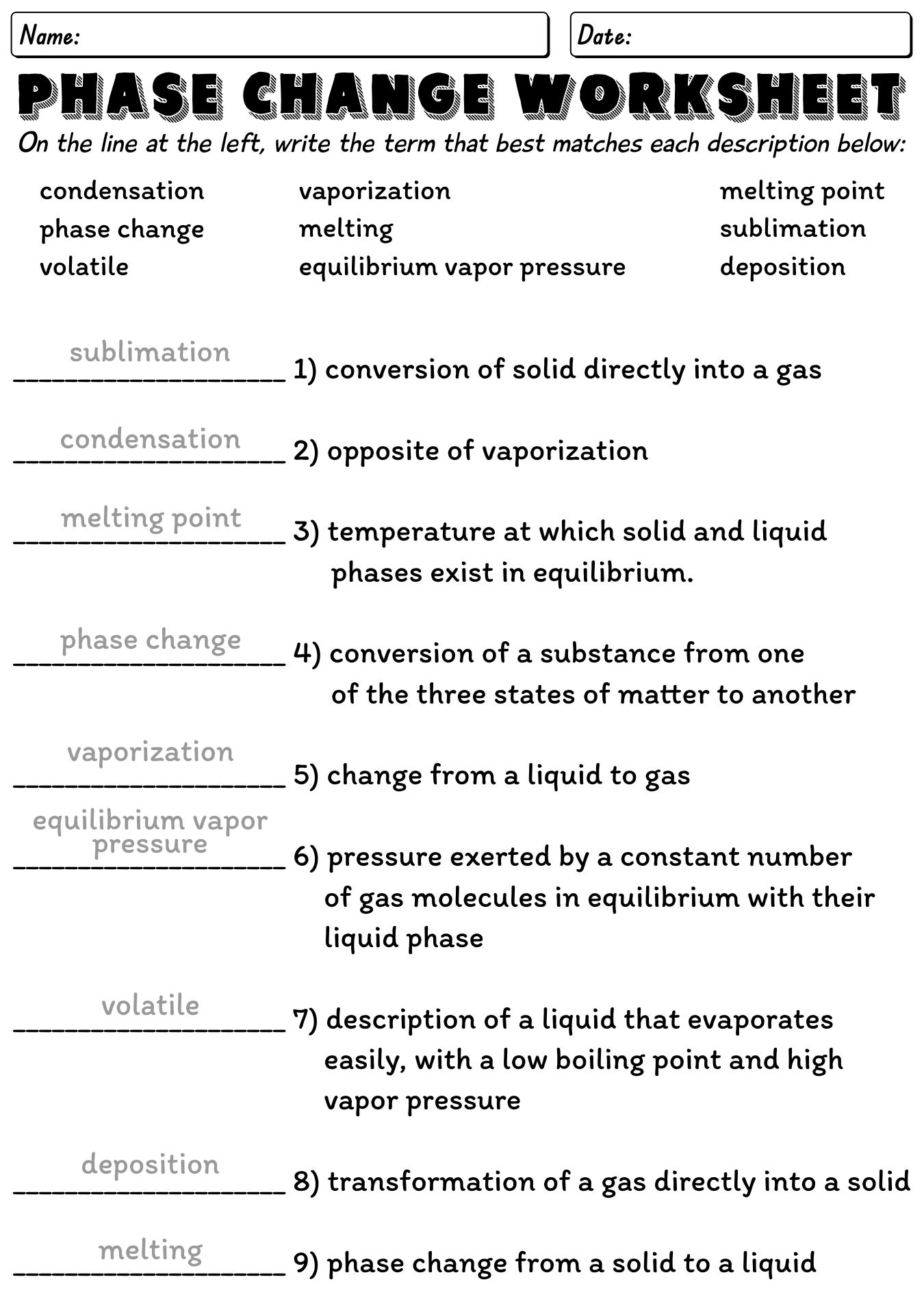
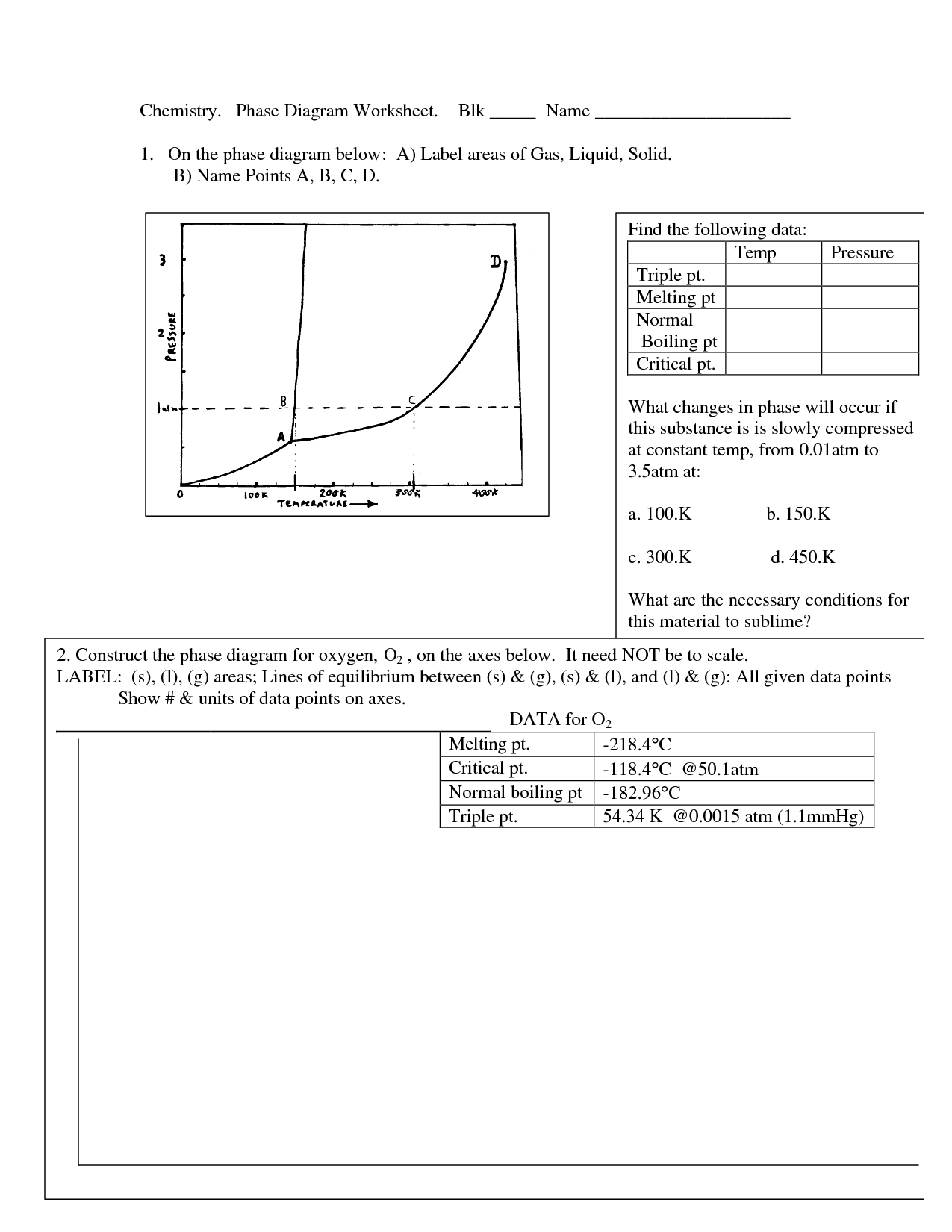
- Chemistry Phase Diagram Worksheet
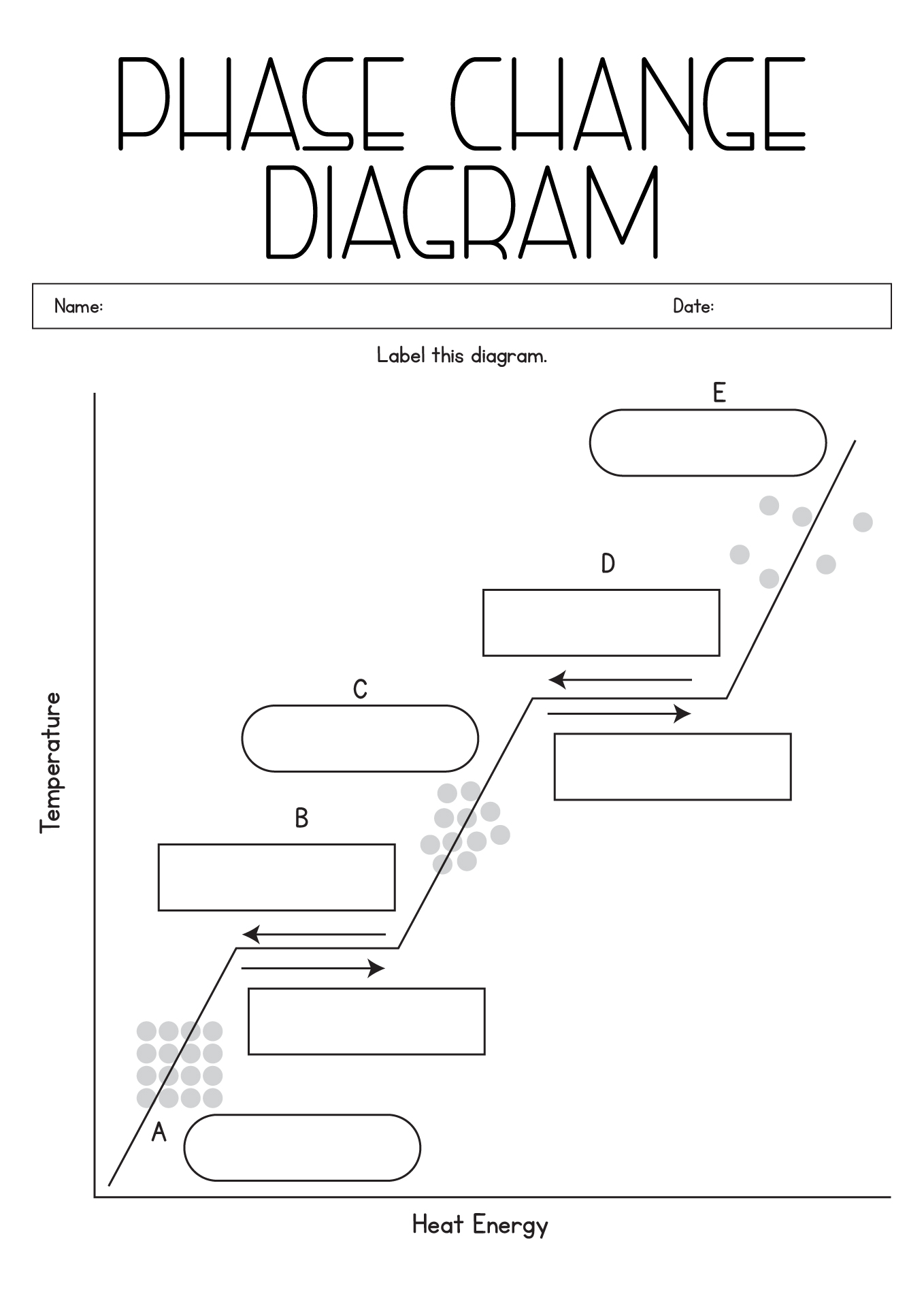
- Label Phase Change Diagram
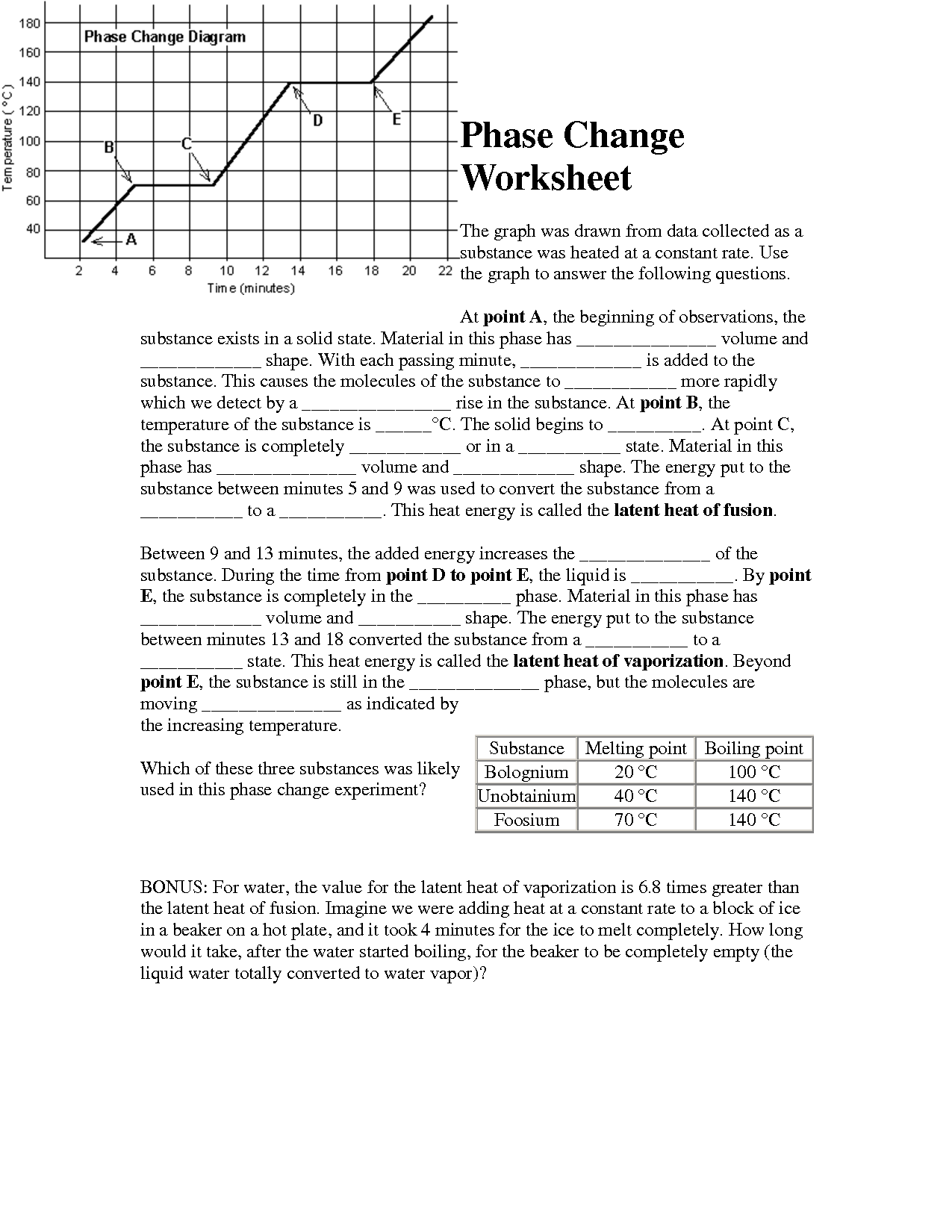
- Phase Change Graph Worksheet
- Science States of Matter Worksheets

More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Spring Clothes Worksheet
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
What is a phase change?
A phase change is a physical change in which a substance transitions from one state of matter to another, such as from a solid to a liquid (melting), from a liquid to a gas (vaporization), or from a gas to a liquid (condensation). During a phase change, the temperature of the substance remains constant as energy is absorbed or released in order to break or form intermolecular forces between particles.
What is melting point?
The melting point is the temperature at which a solid substance changes into a liquid state. At the melting point, the substance's kinetic energy overcomes the intermolecular forces that hold the molecules together in a solid structure, allowing them to move freely and form a liquid.
What happens to particles during melting?
During melting, the particles gain enough thermal energy to overcome the forces holding them in a fixed position, such as intermolecular forces or ionic bonds. As a result, the particles transition from a solid, closely packed arrangement to a more disordered and fluid-like state as they move past each other, allowing the substance to change from a solid to a liquid state.
What is freezing point?
Freezing point is the temperature at which a liquid turns into a solid. At this point, the molecules within the liquid lose enough energy to form a stable structure in a solid state.
What happens to particles during freezing?
During freezing, particles move slower and come closer together as the substance loses thermal energy. As a result, the particles bond together in a more structured manner, forming a solid lattice. This process leads to a decrease in volume and an increase in density of the substance, ultimately transforming it from a liquid to a solid state.
What is vaporization?
Vaporization is the process in which a substance transforms from a liquid state to a gas state, typically due to an increase in temperature or pressure. During vaporization, molecules within the substance gain enough energy to overcome the intermolecular forces holding them together, allowing them to escape into the surrounding environment as a gas. This phase transition is commonly seen in everyday phenomena such as boiling water, where the liquid water turns into steam when heated to its boiling point.
What is the difference between evaporation and boiling?
Evaporation occurs at the surface of a liquid when molecules gain enough kinetic energy to escape as gas, while boiling happens throughout the entire liquid when its vapor pressure equals atmospheric pressure, resulting in bubbles forming and rising to the surface. Evaporation is a slower and gradual process, occurring at any temperature, while boiling is a rapid process that only happens at a specific temperature called the boiling point.
What is condensation?
Condensation is the process by which a gas or vapor turns into a liquid. This occurs when the temperature of the gas or vapor decreases, causing the particles to lose energy and come closer together, forming liquid droplets. It is the opposite of evaporation, where a liquid turns into a gas.
What is sublimation?
Sublimation is the process in which a substance transitions directly from its solid state to its gaseous state without passing through the liquid state. This occurs when the substance's vapor pressure is greater than its atmospheric pressure at a specific temperature, allowing it to evaporate rapidly, such as in the case of dry ice (solid carbon dioxide) turning into carbon dioxide gas without melting into a liquid state.
What is deposition?
Deposition is the process in which sediments, soil, rocks, or other materials are added to a landform or landmass. This can occur through various natural processes such as erosion, weathering, or sedimentation, where the deposited materials accumulate over time to create new land features or alter existing ones.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.

























Comments