Percent Composition and Molecular Formula Worksheet
Are you in need of a comprehensive worksheet that covers percent composition and molecular formulas? If so, you have come to the right place. This worksheet will provide you with all the necessary information and practice problems to master these topics. Whether you are a high school student studying chemistry or a college student taking an introductory chemistry course, this worksheet is designed to suit your needs.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is percent composition?
Percent composition is the percentage by mass of each element in a compound or mixture. It is calculated by dividing the mass of a particular element by the total molar mass of the compound or mixture, and then multiplying the result by 100. This provides insight into the relative abundance of different elements in a sample and is useful for determining the empirical formula of a compound.
How can percent composition be calculated?
Percent composition can be calculated by dividing the mass of a specific element in a compound by the total mass of the compound, and then multiplying the result by 100 to express it as a percentage. This calculation helps in determining the proportion of each element present in a compound by mass.
Why is percent composition important in chemistry?
Percent composition is important in chemistry because it allows us to determine the relative amounts of elements in a compound, which is crucial for understanding the compound's properties and reactivity. By knowing the percentage of each element present, chemists can predict how a compound will behave in reactions, calculate stoichiometry in chemical reactions, and deduce the chemical formula of unknown compounds. This information is essential for designing and synthesizing new materials, understanding chemical reactions, and conducting quantitative analysis in various fields of chemistry.
What information does percent composition provide about a compound?
Percent composition provides the relative amounts of each element present in a compound by expressing the mass percentage of each element in the compound. This information helps determine the chemical formula of the compound and provides insights into its properties and behavior. By knowing the percent composition, one can calculate the empirical formula, molar mass, and potentially predict the compound's reactions and characteristics.
How does percent composition relate to empirical formulas?
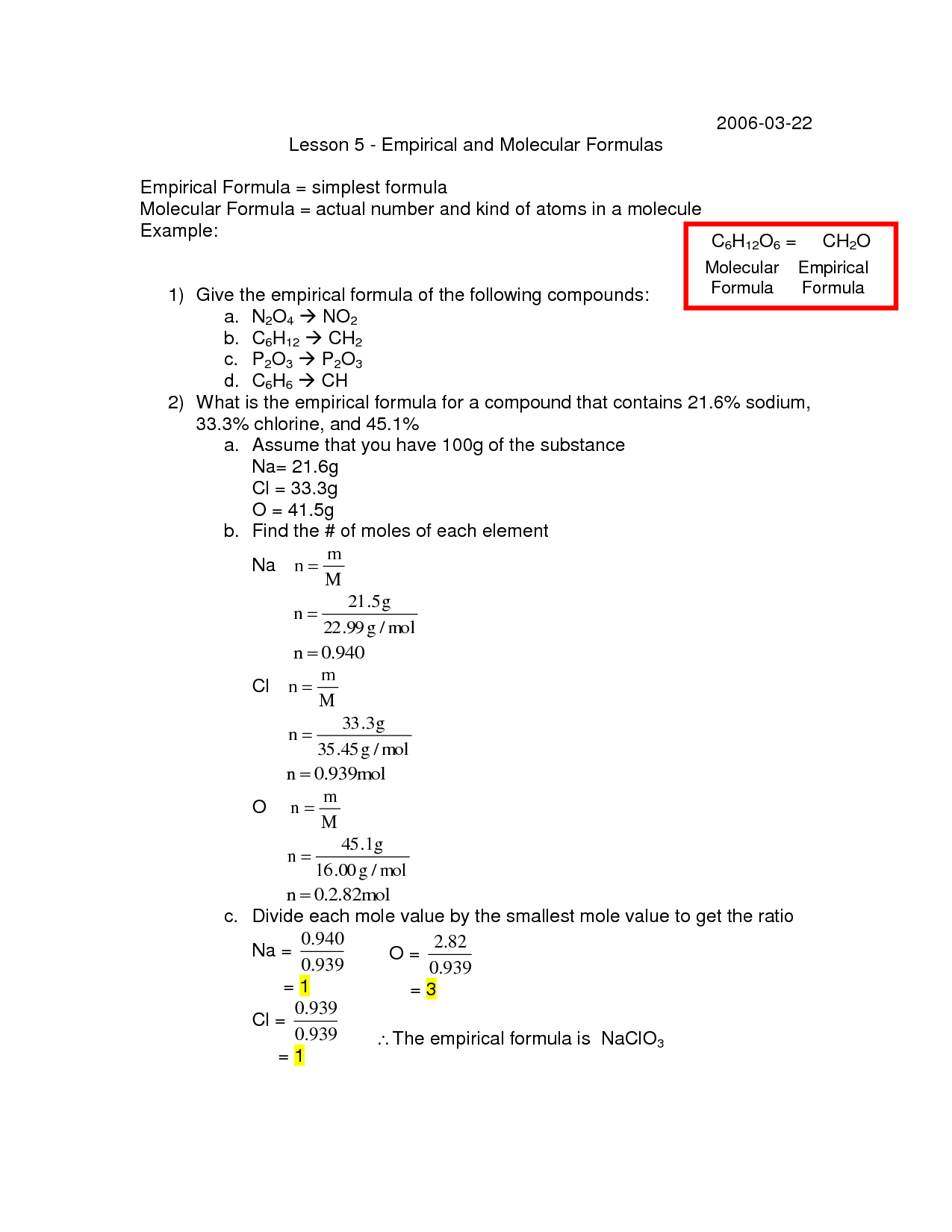
Percent composition is the percentage of each element in a compound by weight. It provides information on the relative amounts of elements present in a compound, which can be used to determine the empirical formula. By converting the percent composition to moles and then to small whole number ratios, the simplest whole number ratio of elements in a compound can be identified, thus giving the empirical formula. The percent composition is essential in calculating empirical formulas and understanding the basic building blocks of a compound.
What is molecular formula and how is it different from empirical formula?
A molecular formula represents the exact number and types of atoms in a molecule, providing the specific arrangement of atoms within a compound. In contrast, an empirical formula shows the simplest ratio of the elements present in a compound, disregarding the actual number of atoms. For example, the molecular formula for hydrogen peroxide is H2O2, which shows it contains two hydrogen atoms and two oxygen atoms per molecule, while its empirical formula is simply HO, indicating the ratio of hydrogen to oxygen atoms in the compound.
How can percent composition be used to determine the molecular formula of a compound?
Percent composition can be used to determine the molecular formula of a compound by first determining the molar mass of the compound from the given percent composition of each element present. Then, by comparing the experimental empirical formula mass to the calculated molar mass, one can determine the ratio between the empirical formula and the molecular formula. By finding the simplest whole-number ratio between the empirical formula and molecular formula, the molecular formula of the compound can be determined.
What are the steps involved in determining the molecular formula using percent composition?
To determine the molecular formula using percent composition, first convert the percentages of each element in the compound into grams. Next, convert the grams of each element into moles using their respective molar masses. Then, divide the moles of each element by the smallest number of moles obtained to determine the mole ratio of the elements in the compound. Finally, use the mole ratios to construct the empirical formula of the compound. To find the molecular formula, compare the experimental molar mass of the compound to the molar mass calculated from the empirical formula and then determine the multiple needed to obtain the correct molar mass, which will give the molecular formula.
How can percent composition and the molecular formula be used to determine the mass of individual elements in a compound?
To determine the mass of individual elements in a compound using percent composition and the molecular formula, one can first calculate the molar mass of the compound using the periodic table to find the individual atomic masses of each element in the compound. Next, one can convert the percentages to masses by assuming a total mass of 100 grams. Using the molar mass and the percentage masses, one can find the number of moles of each element present in the compound. Finally, by multiplying the number of moles of each element by its respective molar mass, one can determine the mass of each individual element in the compound.
What are some practical applications of understanding percent composition and molecular formulas in real-life situations?
Understanding percent composition and molecular formulas is crucial in various real-life situations such as pharmaceuticals, where it helps determine the exact amounts of active ingredients in medications for accurate dosages and effectiveness. In the food industry, it is used to analyze nutritional content and ensure food safety. In environmental science, it aids in studying pollution levels and identifying substances harmful to ecosystems. Additionally, in manufacturing industries, percent composition and molecular formulas are used to control product quality and optimize production processes.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.






























Comments