Identifying Elements Worksheet
If you're a student or teacher in need of a practical and efficient way to reinforce your understanding of key concepts, then this identifying elements worksheet is perfect for you. Designed to help you grasp the fundamentals of various subjects, this worksheet provides a comprehensive overview of the essential elements that make up a specific entity, ensuring that you have a solid foundation for further learning and application.
Table of Images 👆
- Setting Story Elements Worksheets
- Story Element Plot Worksheet
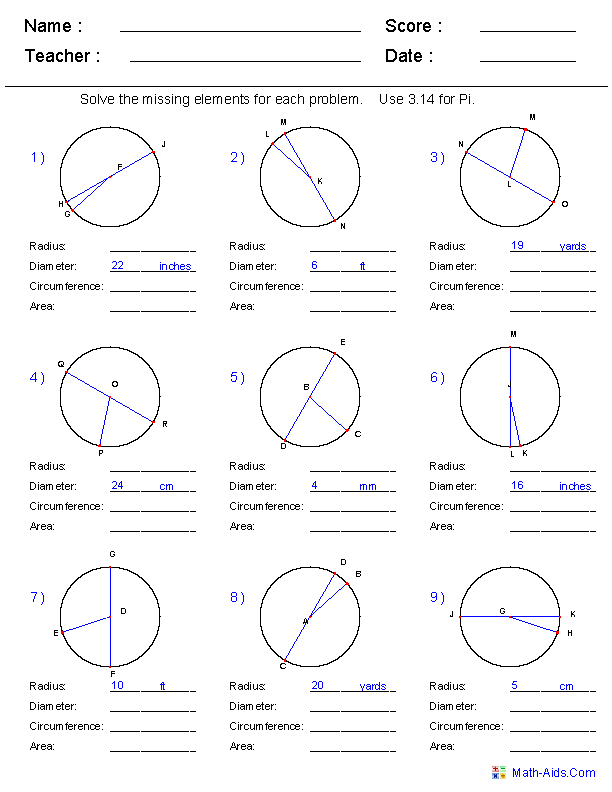
- Radius Diameter Circumference Worksheets
- Science Density Calculations Worksheet
- Character Setting Plot and Theme Worksheets
- Worksheets Identifying Leaves
- Identifying Character Worksheets
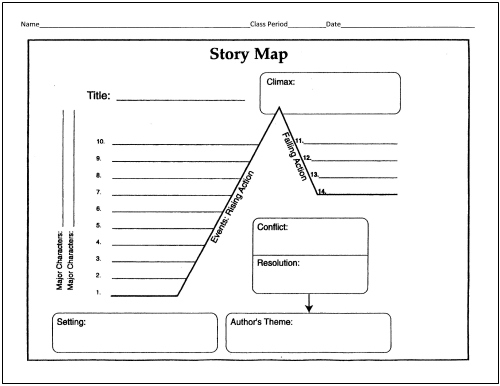
- Story Plot Map Graphic Organizer
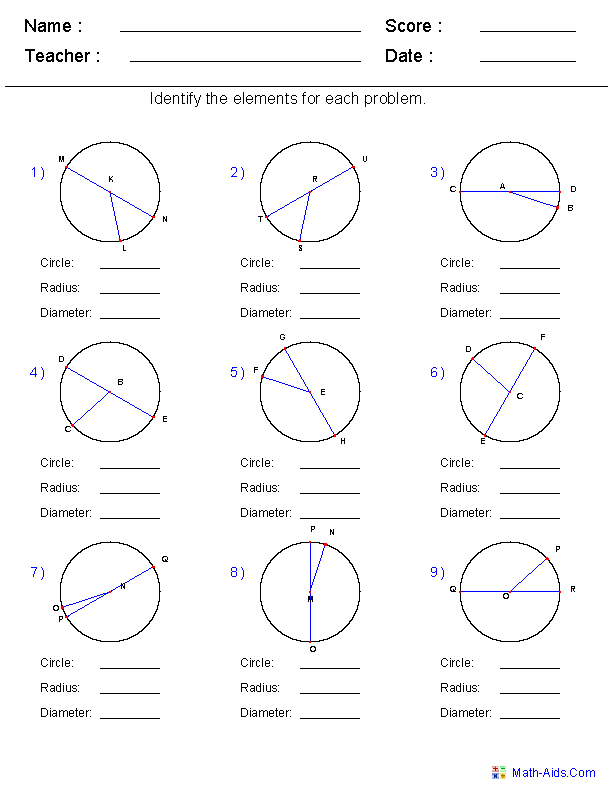
- Geometry Circle Worksheets
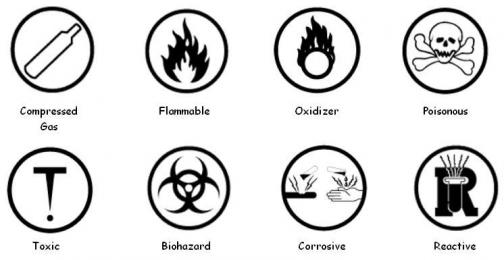
- WHMIS Hazardous Materials Symbols
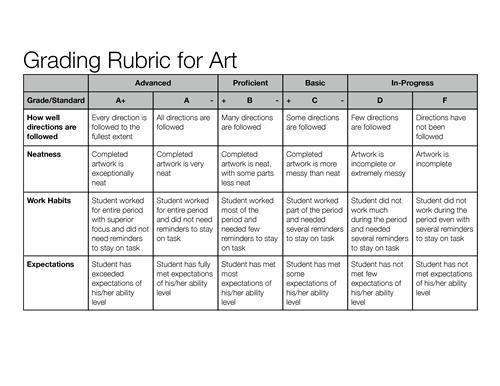
- Art Rubrics for Elementary Students
- English language
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is the atomic number of an element?
The atomic number of an element is the number of protons found in the nucleus of an atom of that element. It is a unique identifier for each element on the periodic table and determines the element's chemical properties.
How can you determine the number of protons in an element?
The number of protons in an element is determined by its atomic number, which is the unique identifier for each element and represents the number of protons in the nucleus of an atom. The atomic number is typically displayed above the element's symbol in the periodic table and can also be found in the element's information on the periodic table.
What is the mass number of an element?
The mass number of an element is the total number of protons and neutrons in the nucleus of an atom. It is an integer value and is used to identify isotopes of an element, as different isotopes of an element have the same number of protons but different numbers of neutrons, resulting in different mass numbers.
How can you calculate the number of neutrons in an element?
To calculate the number of neutrons in an element, you can subtract the atomic number of the element (which represents the number of protons in the nucleus) from the mass number of the element (which represents the total number of protons and neutrons in the nucleus). The mass number is typically listed along with the element symbol on the periodic table. Subtracting the atomic number from the mass number will give you the number of neutrons in the element's nucleus.
What does the symbol of an element represent?
The symbol of an element represents a one- or two-letter abbreviation used to identify a specific chemical element, typically derived from the element's name in English, Latin, or another language. These symbols are used in the periodic table and chemical formulas to provide a concise way to represent elements, facilitating communication in the field of chemistry.
What is the significance of the period number on the periodic table?
The period number on the periodic table indicates the energy level of an element's outermost electrons. Elements in the same period have the same number of electron shells, which affects their chemical properties and reactivity. The period number helps in determining an element's valence electrons, which play a crucial role in its ability to form chemical bonds and react with other elements.
How can you determine the number of electrons in a neutral atom of an element?
To determine the number of electrons in a neutral atom of an element, you would look at the atomic number of the element on the periodic table. The atomic number represents the number of protons in the nucleus of the atom, which is equal to the number of electrons in a neutral atom. So, for any neutral atom of an element, the number of electrons is the same as the atomic number.
How are elements arranged in a group on the periodic table?
Elements in a group on the periodic table are arranged based on similar chemical properties and valence electron configurations. Groups have the same number of valence electrons, which results in similar reactivity and properties among elements within the group. This organization helps to predict the behavior and characteristics of elements based on their placement on the periodic table.
What is the main characteristic of alkali metals?
The main characteristic of alkali metals is that they are highly reactive and easily lose their outermost electron to form a 1+ cation, making them very good reducing agents.
How do elements in the same group on the periodic table typically behave chemically?
Elements in the same group on the periodic table typically behave chemically in similar ways because they have the same number of electrons in their outermost energy level, also known as the valence electrons. This common valence electron configuration gives them similar chemical properties, such as reactivity and the ability to form similar types of compounds. As a result, elements in the same group often exhibit similar trends in their physical and chemical properties.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.




























Comments