Atomic Spectra Worksheet 1 Answers
In this blog post, we will be providing the answers to Atomic Spectra Worksheet 1. This worksheet is designed for students who are studying atomic spectra and are interested in testing their knowledge on the subject. By providing the answers, we aim to assist and reinforce the understanding of this intricate topic.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is atomic spectra?
Atomic spectra are the specific wavelengths of light emitted or absorbed by atoms when they undergo transitions between energy states. These spectra are unique to each element and are useful for identifying elements and studying their behavior. The emission spectrum consists of discrete lines of light representing the energy released as electrons move to lower energy levels, while the absorption spectrum displays dark lines where specific wavelengths of light are absorbed by the atoms.
How are atomic spectra produced?
Atomic spectra are produced when atoms absorb or emit light. When an atom absorbs energy, such as heat or electricity, its electrons become excited and move to higher energy levels. As these electrons return to their original energy levels, they emit light in the form of discrete wavelengths that correspond to the differences in energy levels. These wavelengths create a spectrum unique to each element, known as its atomic spectrum, which can be used to identify the element and provide information about its structure.
What causes an atom to emit light at specific wavelengths?
An atom emits light at specific wavelengths when its electrons move between energy levels or orbitals within the atom. When an electron transitions from a higher energy level to a lower one, it emits light in the form of photons. The specific wavelength of the emitted light is determined by the difference in energy levels between the two states, and this difference corresponds to a specific color of light. This phenomenon is known as atomic emission and is governed by the unique electronic structure of the atom.
What is the relationship between atomic energy levels and spectral lines?
The relationship between atomic energy levels and spectral lines is that the arrangement of energy levels within an atom determines the specific wavelengths of light that are emitted or absorbed by that atom, producing spectral lines. When an atom undergoes a transition between energy levels, it emits or absorbs energy in the form of electromagnetic radiation, creating spectral lines at certain frequencies. These spectral lines provide valuable information about the structure and properties of the atom, and they are used in various scientific fields, such as spectroscopy, to study the composition and behavior of matter.
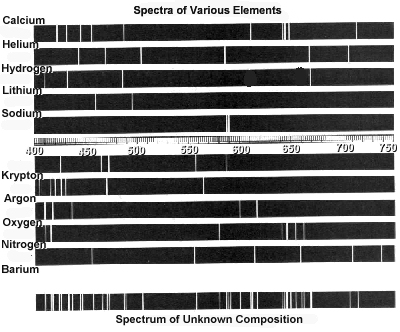
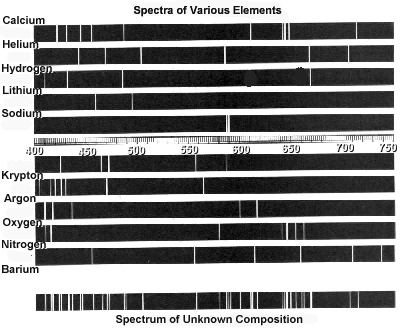
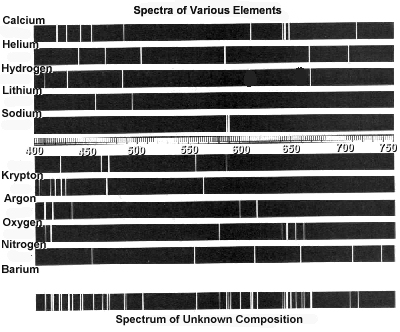
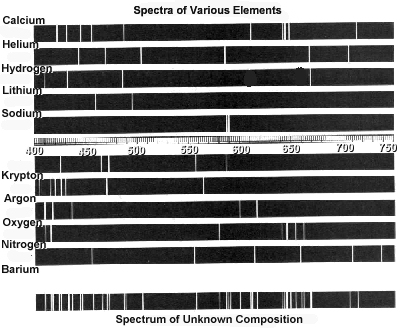
How can atomic spectra help identify elements?
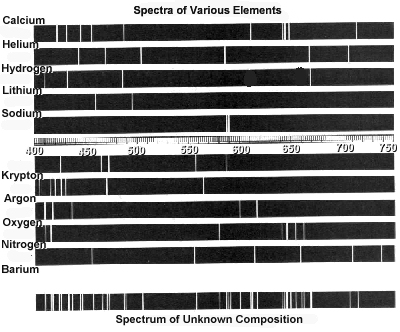
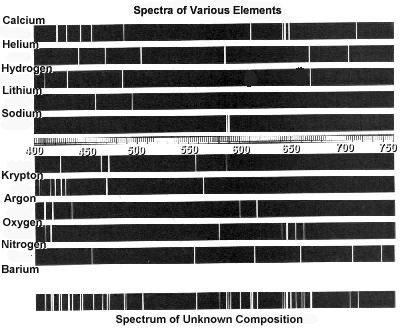
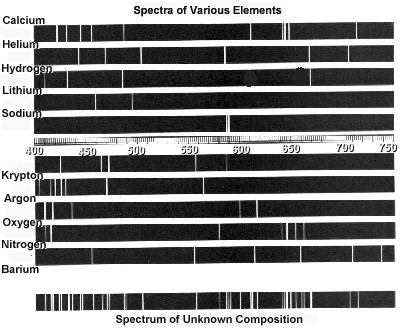
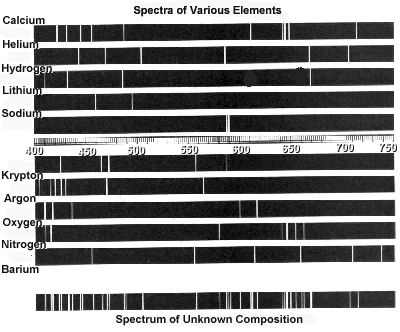
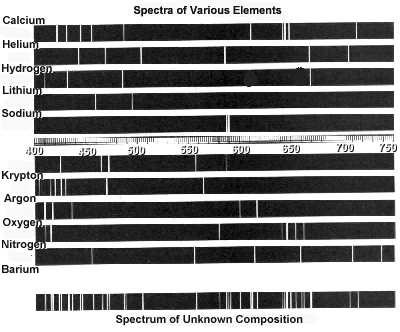
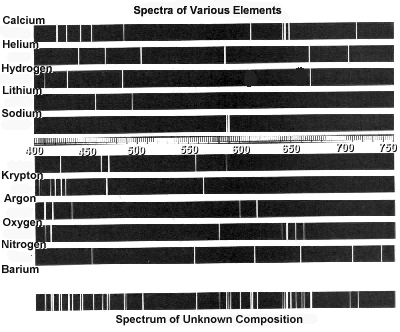
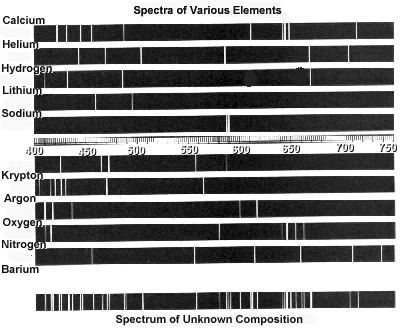
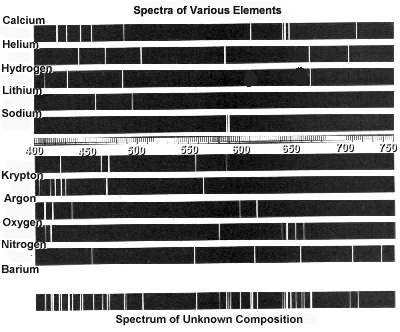
Atomic spectra can help identify elements by providing a unique "fingerprint" of the light emitted or absorbed by an element when its electrons move between energy levels. Each element has a specific set of energy levels and transitions, leading to distinct spectral lines on the spectrum. By comparing these spectral lines to known patterns, scientists can match them to elements and determine their presence in a sample, making atomic spectra a powerful tool for identifying elements in a given substance.
What is the difference between absorption and emission spectra?
Absorption spectra show the wavelengths of light that are absorbed by a substance, while emission spectra show the wavelengths of light that are emitted by a substance. In absorption spectra, dark lines represent the wavelengths that are absorbed by the material, whereas in emission spectra, bright lines represent the wavelengths that are emitted. Essentially, absorption spectra reveal the colors of light absorbed by a material, whereas emission spectra reveal the colors of light emitted by a material.
How does the Bohr model explain atomic spectra?
The Bohr model explains atomic spectra by proposing that electrons in an atom can only occupy specific energy levels. When an electron moves from one energy level to another, it emits or absorbs energy in the form of electromagnetic radiation, such as light. This emission or absorption of energy results in the observed discrete lines in an atom's spectrum, as each line corresponds to a specific energy difference between electron energy levels. The Bohr model successfully explains why atoms emit or absorb light at specific wavelengths, providing a foundation for understanding atomic spectra.
What are the primary types of atomic spectra?
The primary types of atomic spectra are emission spectra and absorption spectra. Emission spectra are produced when atoms emit light as electrons move from higher to lower energy levels, resulting in distinct lines of color. Absorption spectra are created when atoms absorb light at specific wavelengths, causing dark lines to appear in the spectrum where light is absorbed. These spectral lines provide valuable information about the energy levels and composition of atoms.
How do electron transitions relate to spectral lines?
Electron transitions refer to the movement of electrons between different energy levels within an atom. When an electron moves from a higher energy level to a lower one, it emits a photon of light with a specific energy corresponding to the energy difference between the two levels. This emitted light appears as a spectral line in the electromagnetic spectrum, with each line corresponding to a specific transition. Therefore, electron transitions directly relate to spectral lines by revealing the distinct energy levels within an atom through the emission or absorption of light at specific wavelengths.
Why are atomic spectra important in astronomy?
Atomic spectra are important in astronomy because they provide critical information about the composition, temperature, density, and motion of distant astronomical objects such as stars, galaxies, and nebulae. By studying the specific wavelengths of light emitted or absorbed by atoms in these objects, astronomers can determine their chemical makeup, temperature, and velocity. This helps in understanding the physical processes at play in these celestial bodies and can provide insights into their evolution and behavior. Additionally, analyzing atomic spectra can also reveal information about the conditions and history of the universe as a whole.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.
























Comments