Reaction Types Worksheet
Looking for a helpful tool to reinforce your understanding of reaction types? Look no further than this Reaction Types Worksheet. Designed for students studying chemistry or those interested in learning more about chemical reactions, this worksheet covers a range of reaction types and provides practice problems to test your knowledge.
Table of Images 👆
- Chemical Reaction Types Worksheet
- Types of Chemical Reactions Worksheet Answer Key
- Predicting Products of Chemical Reactions Worksheet Answers
- Chemical Reactions Worksheet
- Balance the Following Equations Worksheet
- Balancing Reaction Worksheet and Types
- Types of Reactions Worksheet Answer Key
- Balanced Equation for Silver Nitrate and Sodium Chloride
- Reaction Types Worksheet Answer Key
- Chemistry Worksheet Answer Keys
- Printable Cloud Type Worksheets
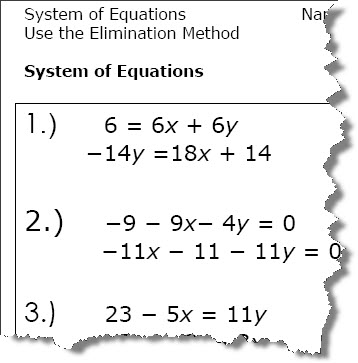
- Systems of Equations Elimination Method Worksheet
- Chapter 8 Covalent Bonding Worksheet Answer Key
- Balancing Chemical Equations Worksheet Answer Key
- Physical vs Chemical Change Worksheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is a synthesis reaction?
A synthesis reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex substance. This process typically involves the formation of a single product from multiple reactants, with the combination of elements or compounds to create new chemical bonds, and often requires energy input to overcome activation energy barriers.
Describe a decomposition reaction.
A decomposition reaction is a type of chemical reaction where a single compound breaks down into two or more simpler substances. This process is usually initiated by heat, light, electricity, or a chemical reaction. A common example of a decomposition reaction is the breakdown of hydrogen peroxide into water and oxygen gas.
What is a single replacement reaction?
A single replacement reaction is a chemical reaction in which an element replaces another element in a compound. This occurs when a more reactive element displaces a less reactive element from its compound, resulting in a new compound and a different element being freed.
Give an example of a double replacement reaction.
An example of a double replacement reaction is when aqueous solutions of silver nitrate (AgNO3) and sodium chloride (NaCl) are mixed together, resulting in the formation of solid silver chloride (AgCl) and sodium nitrate (NaNO3) in solution. The silver cations from silver nitrate switch places with the sodium cations from sodium chloride, leading to the formation of the two new compounds.
Explain what happens in a combustion reaction.
In a combustion reaction, a fuel combines with an oxidizer (usually oxygen) to produce heat, light, and usually other products such as carbon dioxide and water vapor. The fuel undergoes rapid oxidation, releasing energy in the form of heat and light. This reaction is exothermic, meaning it releases more energy than it consumes. Combustion reactions are the basis for many everyday processes, including burning wood for heat, gasoline in engines for transportation, and natural gas for cooking.
Describe an acid-base reaction.
An acid-base reaction is a chemical reaction that occurs between an acid, which donates protons, and a base, which accepts protons. When an acid and a base react, they form water and a salt. The acid donates a proton to the base, forming the conjugate base of the acid and the conjugate acid of the base. This reaction results in the neutralization of the acid and base, producing a salt and water as the products. Acid-base reactions are fundamental in chemistry and play a crucial role in various chemical processes and biological systems.
What is an oxidation-reduction (redox) reaction?
An oxidation-reduction (redox) reaction is a type of chemical reaction in which one substance loses electrons (oxidation) while another gains electrons (reduction). This transfer of electrons results in a change in the oxidation states of the reacting species. Redox reactions are key in many important processes such as the generation of energy in our cells (cellular respiration) and corrosion of metals.
Give an example of a precipitation reaction.
One example of a precipitation reaction is the reaction between silver nitrate (AgNO3) and sodium chloride (NaCl) solutions, which forms a solid precipitate of silver chloride (AgCl) as shown in the equation: AgNO3 (aq) + NaCl (aq) ? AgCl (s) + NaNO3 (aq).
Explain what happens in a neutralization reaction.
In a neutralization reaction, an acid and a base react to form water and a salt. The acid donates a hydrogen ion (H+) and the base donates a hydroxide ion (OH-) to form water (H2O). The remaining ions then combine to form a salt. The overall result of the neutralization reaction is the formation of water and a salt, with the acidity and basicity of the initial reactants cancelling each other out and resulting in a neutral pH.
Describe a displacement reaction.
A displacement reaction is a type of chemical reaction where a more reactive element displaces a less reactive element from its compound. This occurs when two reactants compete for the same partner in a chemical reaction, with the more reactive element pushing out the less reactive element to form a new compound. The displaced element is then released as a separate product. This type of reaction is commonly seen in metals reacting with metal salts or halogens displacing each other in solutions.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.

































Comments