Periodic Table Worksheet Answers
Are you a student or teacher searching for a reliable resource to reinforce your understanding of the periodic table? Look no further. In this blog post, we will explore how worksheets can enhance your learning experience by providing comprehensive answers to your periodic table questions. Whether you are studying chemistry, preparing for an exam, or simply curious about the elements, these worksheets will serve as a valuable tool in your educational journey.
Table of Images 👆
- Periodic Table Worksheet Answer Key
- Periodic Table Puns Worksheet Answers
- Chemistry Periodic Table Worksheet Answers
- Periodic Table Basics Worksheet Answer Key
- Periodic Table Trends Worksheet Answer Key
- Periodic Table Scavenger Hunt Worksheet Answers
- Periodic Table Worksheet Key
- Atomic Structure Worksheet and Periodic Table
- Periodic Trends Worksheet Answers
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is the Periodic Table?
The Periodic Table is a tabular arrangement of all known chemical elements, organized by their atomic number, electron configuration, and recurring chemical properties. It provides a systematic way to understand and predict the behavior of elements based on their position within the table. The Periodic Table is a fundamental tool in chemistry and is used to study the relationships between different elements and their compounds.
How many elements are represented in the Periodic Table?
There are 118 elements represented in the Periodic Table.
What is the significance of the atomic number in the Periodic Table?
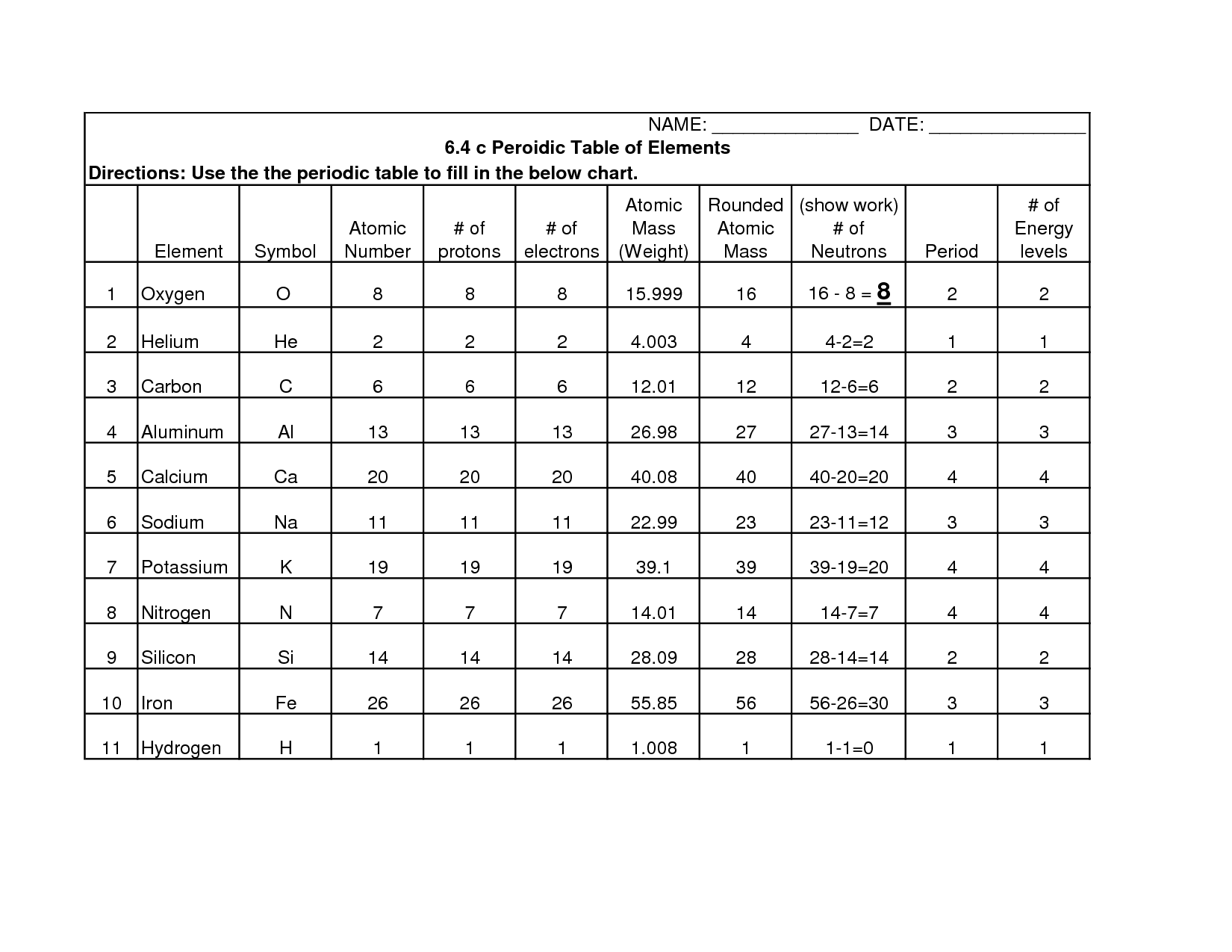
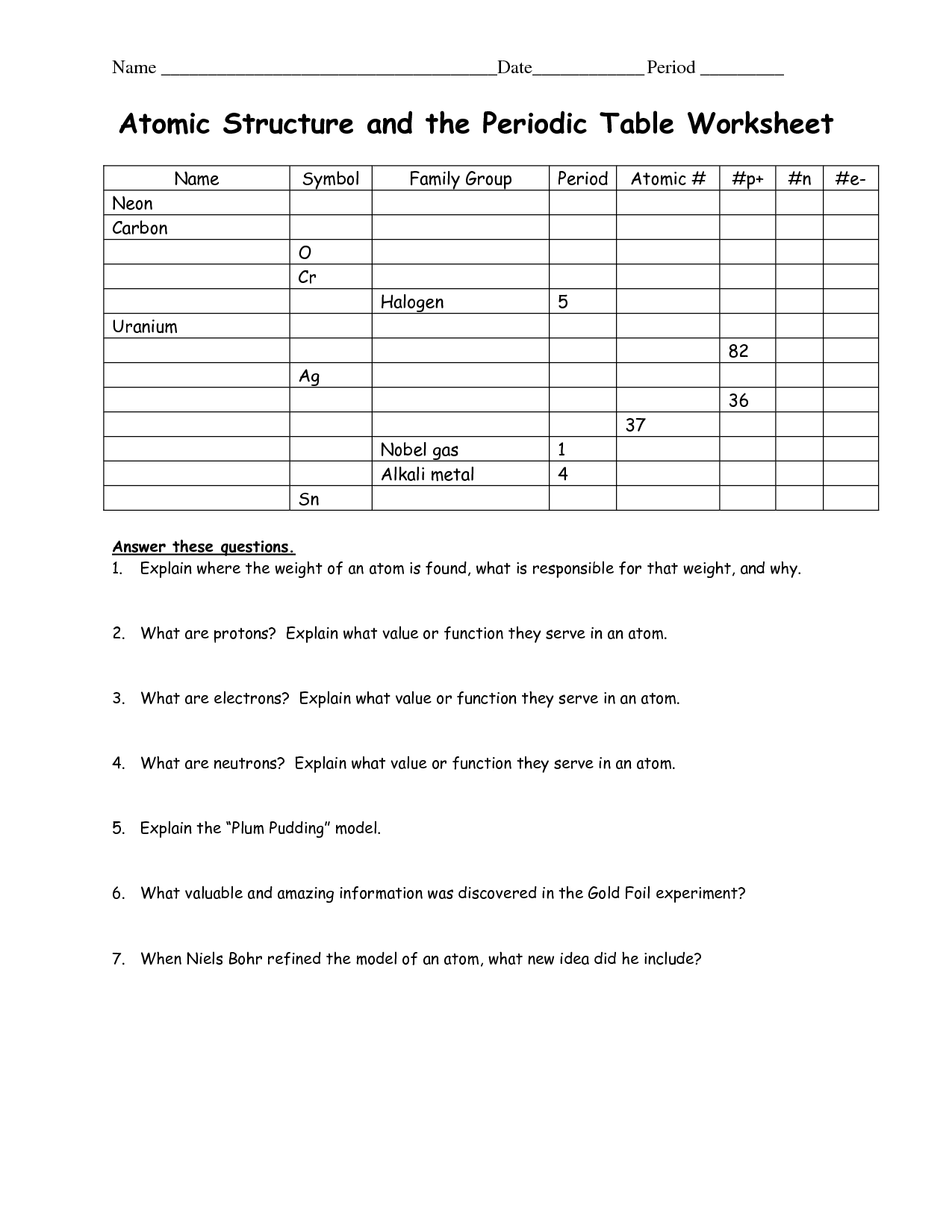
The atomic number in the Periodic Table represents the number of protons in the nucleus of an atom, which determines the element's identity. It also influences an element's chemical properties, as it dictates the number of electrons in a neutral atom. Thus, the atomic number is crucial for organizing elements in the Periodic Table based on their unique properties and providing a systematic way to study and predict their behavior.
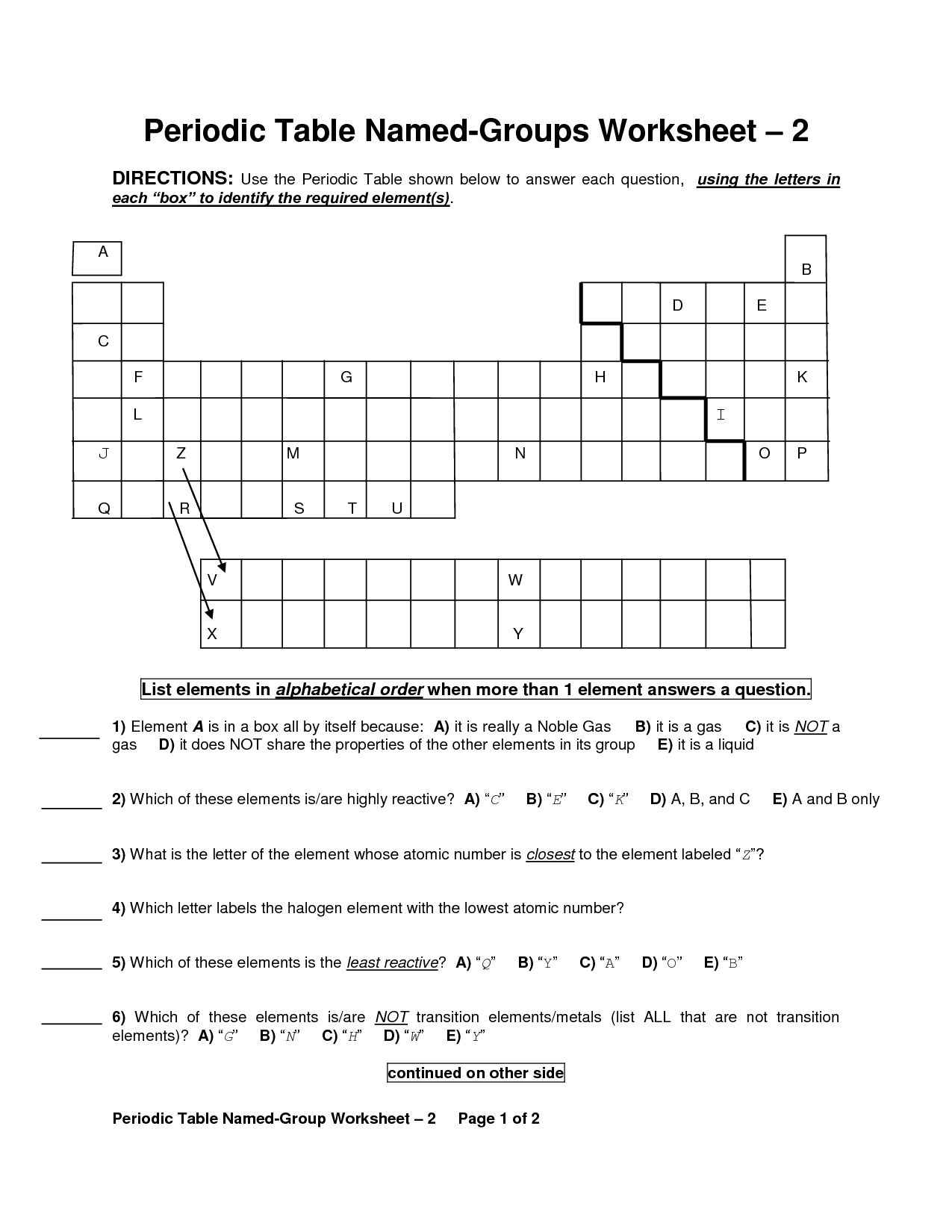
What are the periods and groups in the Periodic Table?
The Periodic Table is organized into periods, which are the horizontal rows, and groups, which are the vertical columns. There are seven periods and 18 groups in the Periodic Table. Each period represents the energy level of the elements, while each group shares similar chemical properties due to having the same number of valence electrons.
How are elements arranged in the Periodic Table?
Elements in the Periodic Table are arranged by atomic number, which is the number of protons in the nucleus of an atom. The table is organized in periods (rows) and groups (columns) based on similarities in chemical properties. Elements in the same group have similar characteristics due to the arrangement of their electrons in the outer shell, while elements in the same period have the same number of electron shells. The Periodic Table helps scientists predict properties of elements and their behavior in chemical reactions.
What is the difference between metals, nonmetals, and metalloids?
Metals are elements that are typically shiny, malleable, and good conductors of heat and electricity, while nonmetals are elements that are typically dull, brittle, and poor conductors of heat and electricity. Metalloids have properties that are intermediate between metals and nonmetals, such as being semiconductors. These categories are based on the physical and chemical properties of elements on the periodic table.
What is a chemical symbol and how is it used in the Periodic Table?
A chemical symbol is a unique abbreviation used to represent elements in the Periodic Table. Each element is assigned a specific symbol, usually derived from its name in English or Latin. These symbols are typically one or two letters long, with the first letter always capitalized. Chemical symbols are an essential part of the Periodic Table as they provide a simple and consistent way to identify and reference elements in various chemical reactions, equations, and analyses.
How can you determine the properties of an element based on its position in the Periodic Table?
The properties of an element can be determined based on its position in the Periodic Table by looking at its atomic number, group number, and period number. The atomic number tells you the number of protons in the nucleus of an atom and is unique to each element, influencing its chemical behavior. The group number indicates the number of valence electrons the element has, which affects its reactivity and bonding behavior. The period number signifies the energy levels or electron shells in which the element's electrons are arranged, impacting its physical and chemical properties. By considering these factors, you can gather insights into an element's characteristics and behavior.
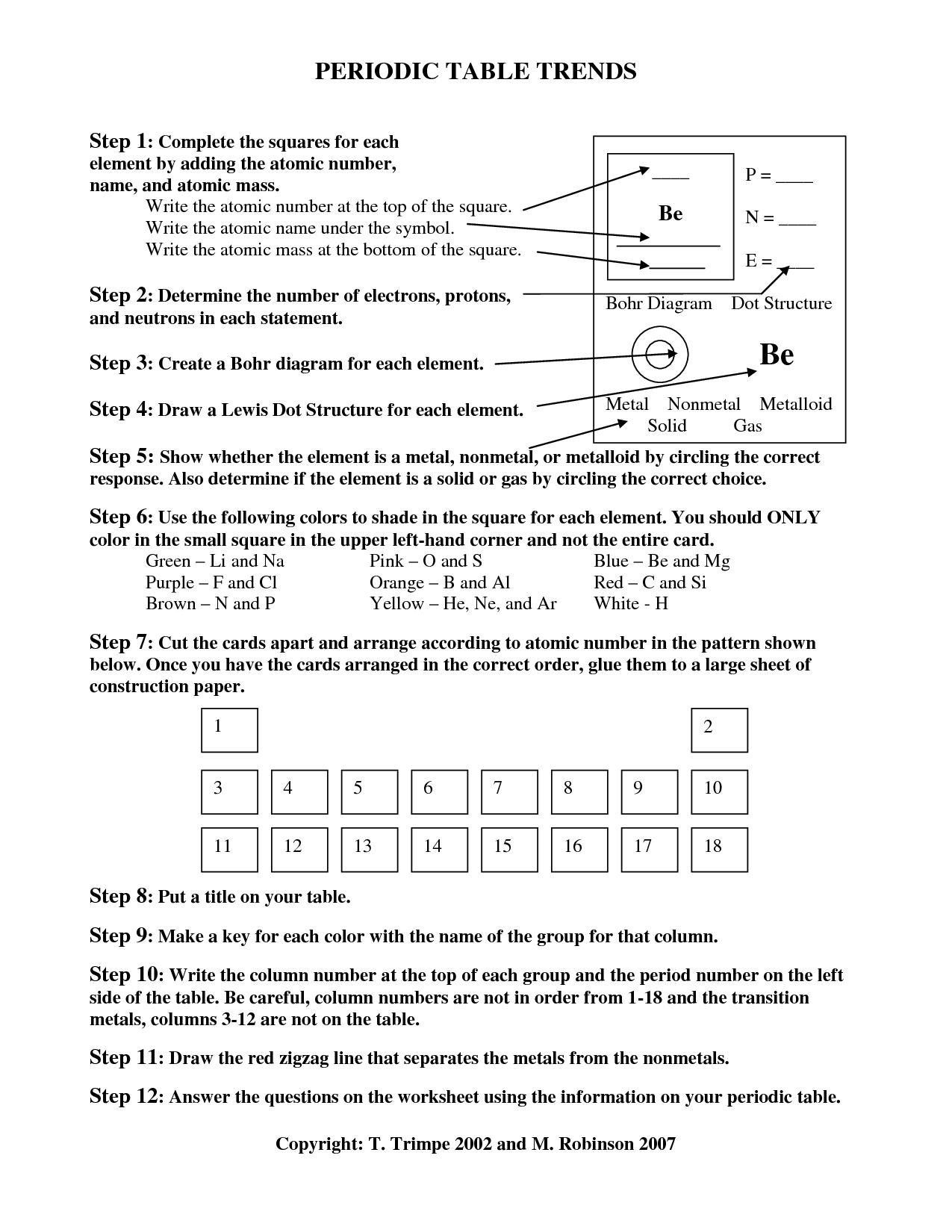
What is the relationship between periodic trends and the arrangement of elements in the Periodic Table?
Periodic trends are patterns in properties of elements that change predictably as you move across periods and down groups in the Periodic Table. These trends are a result of the arrangement of elements in the Periodic Table where elements are organized in order of increasing atomic number, with elements with similar properties grouped together. The Periodic Table's arrangement allows us to easily identify and understand the trends in properties such as atomic size, ionization energy, electronegativity, and metallic character.
How does the Periodic Table help in predicting the behavior and characteristics of elements?
The Periodic Table helps predict the behavior and characteristics of elements by organizing them based on their atomic number and chemical properties. Elements in the same group tend to have similar chemical properties due to their shared electron configurations and valence electrons, which influences how they react with other substances. By knowing an element's group and period on the Periodic Table, scientists can make educated predictions about its reactivity, bonding tendencies, and physical properties. This organized system allows for the classification and understanding of the vast array of elements and their behavior in chemical reactions.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



























Comments