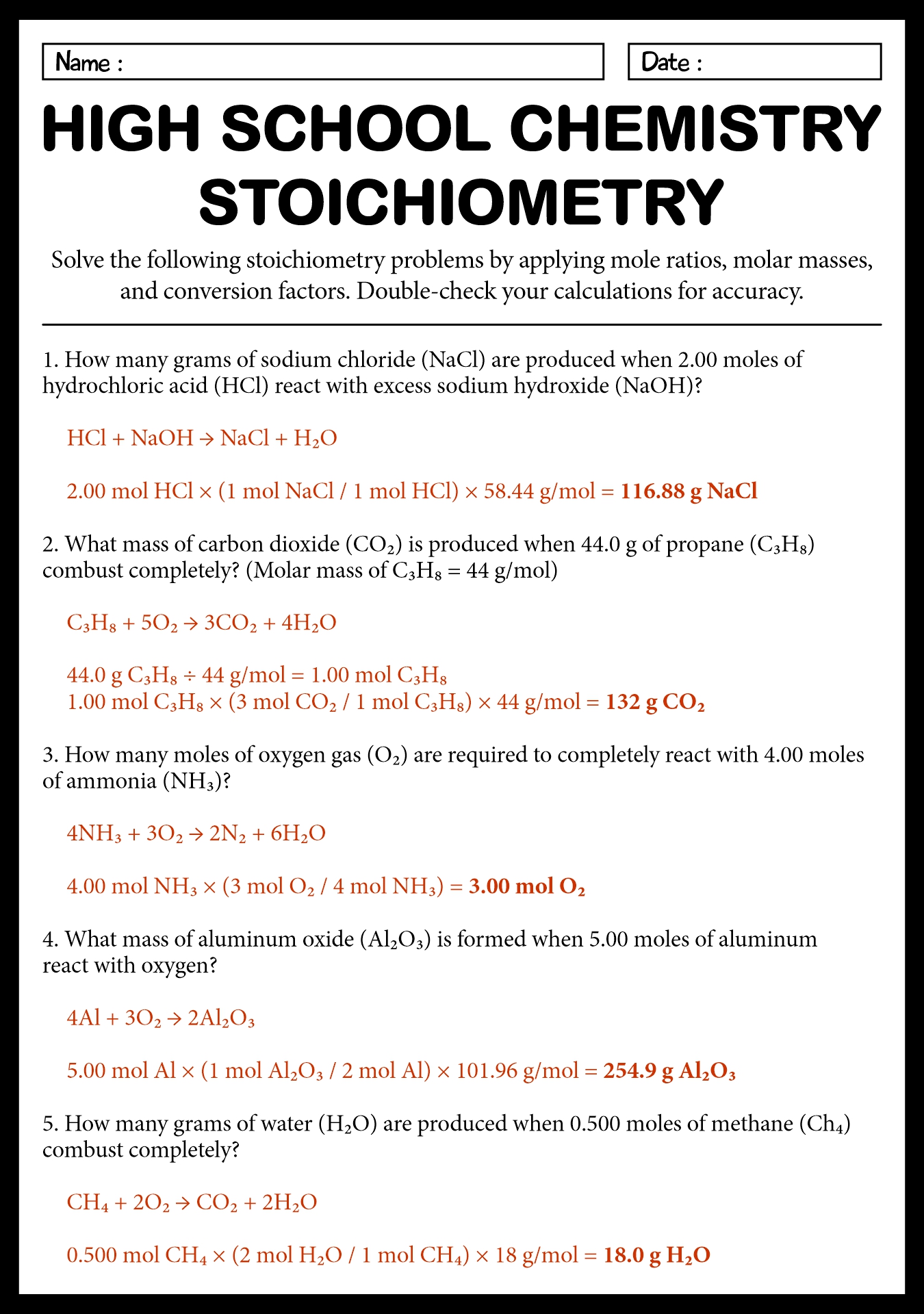
Chemistry Stoichiometry Worksheet Answer Key
Are you a chemistry student looking for a comprehensive and reliable resource to practice your stoichiometry skills? Look no further! We have created a Chemistry Stoichiometry Worksheet Answer Key that is perfect for you.
Table of Images 👆
- Stoichiometry Worksheet Answer Key
- Chemistry Stoichiometry Worksheet Answers
- Mole Conversion Worksheet Answer Key
- Stoichiometry Worksheet Answers
- Stoichiometry Worksheet Answer Key
- Chemistry Worksheet Answer Keys
- Molar Mass Moles and Avogadros Number Worksheet
- Chemistry Stoichiometry Worksheet Solution Guide
- Stoichiometry Problem Solving Worksheet Answer Key
- High School Chemistry Stoichiometry Worksheet Key
- Stoichiometry Conversion Worksheet With Correct Answers
- Basic Stoichiometry Worksheet with Answer
- General Chemistry Stoichiometry Worksheet Answer Key

More Chemistry Worksheets
Chemistry Lab Equipment WorksheetChemistry Stoichiometry Worksheet Answer Key
Chemistry Conversion Factors Worksheet
Fun Chemistry Worksheets
What is the definition of stoichiometry?
Stoichiometry is a branch of chemistry that deals with the calculation of the quantities of reactants and products involved in chemical reactions based on the laws of conservation of mass and energy. It involves determining the proportions in which elements combine to form compounds and the quantities of reactants needed to produce a particular amount of product in a chemical reaction.
Explain the importance of stoichiometry in chemical reactions.
Stoichiometry is crucial in chemical reactions as it allows us to accurately predict the amount of reactants needed and the amount of products formed. By balancing chemical equations using stoichiometric calculations, we can optimize the efficiency of reactions, minimize waste, and ensure the desired product is obtained. It also provides valuable information for scaling up reactions in industrial processes and enables scientists to understand the underlying principles of chemical reactions, ultimately helping in the development of new materials and technologies.
How is stoichiometry used to balance chemical equations?
Stoichiometry is used to balance chemical equations by comparing the number of atoms of each element on both sides of the equation. Coefficients are added in front of the chemical formulas to ensure that the same number of atoms of each element is present on both sides of the equation, thus satisfying the law of conservation of mass. Calculations involving molar ratios and mole-to-mole relationships are used to determine the correct coefficients needed to balance the equation.
What are the steps involved in performing stoichiometric calculations?
To perform stoichiometric calculations, first, balance the chemical equation for the reaction. Then, determine the mole ratio between the reactants and products. Next, convert given quantities of reactants or products into moles using their molar masses. Use the mole ratio from the balanced equation to calculate the moles of the desired substance. Finally, convert the moles back into the desired units (grams or liters) if needed. Repeat these steps for each substance involved in the reaction to complete the stoichiometric calculations.
How is the concept of moles used in stoichiometry?
In stoichiometry, the concept of moles is used to determine the relative quantities of reactants and products involved in a chemical reaction. By converting the quantities of substances into moles, it allows for the establishment of mole ratios, which are then used to calculate the amount of one substance needed to react completely with another. This helps in predicting the amount of products formed or the amount of reactants needed for a desired reaction, making mole calculations an essential part of stoichiometry.
Discuss how stoichiometry can be applied to determine the limiting reactant in a reaction.
Stoichiometry can be applied to determine the limiting reactant in a reaction by comparing the amount of each reactant involved in a chemical equation to calculate which one will be fully consumed first. By converting the mass or moles of each reactant to the same unit, one can use the stoichiometric coefficients from the balanced equation to see which reactant will produce the least amount of product. The reactant that produces the least amount of product is considered the limiting reactant because it restricts the amount of product that can be formed. This information is crucial in determining the maximum amount of product that can be obtained and is essential for optimizing reaction conditions.
Describe the concept of percent yield and its significance in stoichiometry.
Percent yield is a measurement used in chemistry to determine how efficiently a reaction produces the desired product compared to the theoretical yield. It is calculated by dividing the actual yield by the theoretical yield, then multiplying by 100 to get a percentage. Percent yield provides valuable information about the efficiency of a chemical reaction and helps chemists to identify any potential sources of error or inefficiency in their experimental procedures. In stoichiometry, percent yield is crucial for determining the success of a reaction and ensuring that the correct amount of products are obtained based on the balanced equation.
Explain how stoichiometry can be used to calculate the theoretical yield of a reaction.
Stoichiometry involves using the balanced chemical equation of a reaction to determine the quantitative relationship between reactants and products. By converting the amounts of reactants used in a reaction to moles, one can then use the stoichiometric ratios to calculate the theoretical yield of a product. This calculation is based on the limiting reactant, which is the reactant that is completely consumed and determines the maximum amount of product that can be formed. The theoretical yield represents the expected amount of product that could be obtained under ideal conditions, assuming the reaction goes to completion and no side reactions occur.
How does stoichiometry help in determining the molar ratios between reactants and products in a chemical equation?
Stoichiometry helps in determining the molar ratios between reactants and products in a chemical equation by using the balanced equation as a guide. The coefficients of the balanced equation represent the mole-to-mole ratios of the reactants and products. By applying stoichiometric calculations, such as molar conversions and mole ratios, one can determine the exact amount of reactants needed and products produced in a chemical reaction based on the balanced equation, allowing for precise measurements and predictions in chemical reactions.
Discuss the applications of stoichiometry in various fields of chemistry.
Stoichiometry plays a crucial role in various fields of chemistry by helping to determine the quantities of reactants and products involved in chemical reactions. In analytical chemistry, stoichiometry is used to accurately measure and analyze the composition of substances. In environmental chemistry, it is utilized to understand the chemical processes involved in pollution and environmental degradation. In industrial chemistry, stoichiometry is critical for designing efficient production processes and optimizing reaction yields. Lastly, in biochemistry, stoichiometry is applied to study metabolic pathways and the interactions of different molecules within biological systems. Overall, stoichiometry is a fundamental tool in chemistry that has broad applications across different disciplines.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



























Comments