Worksheets Compound and Elements Printable
Are you on the lookout for worksheets that can help your students master compound elements? If so, you've come to the right place. In this blog post, we will explore a variety of printable worksheets that focus on compounds and elements, designed to enhance the learning experience for students in middle and high school science classes. Whether you are a teacher searching for additional resources or a parent looking to support your child's learning at home, these worksheets will provide valuable practice and reinforcement for understanding the world of compounds and elements.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is a compound?
A compound is a substance made up of two or more elements that are chemically bonded together in a fixed ratio. The properties of a compound are different from those of its individual elements, and the components of a compound can only be separated by a chemical reaction.
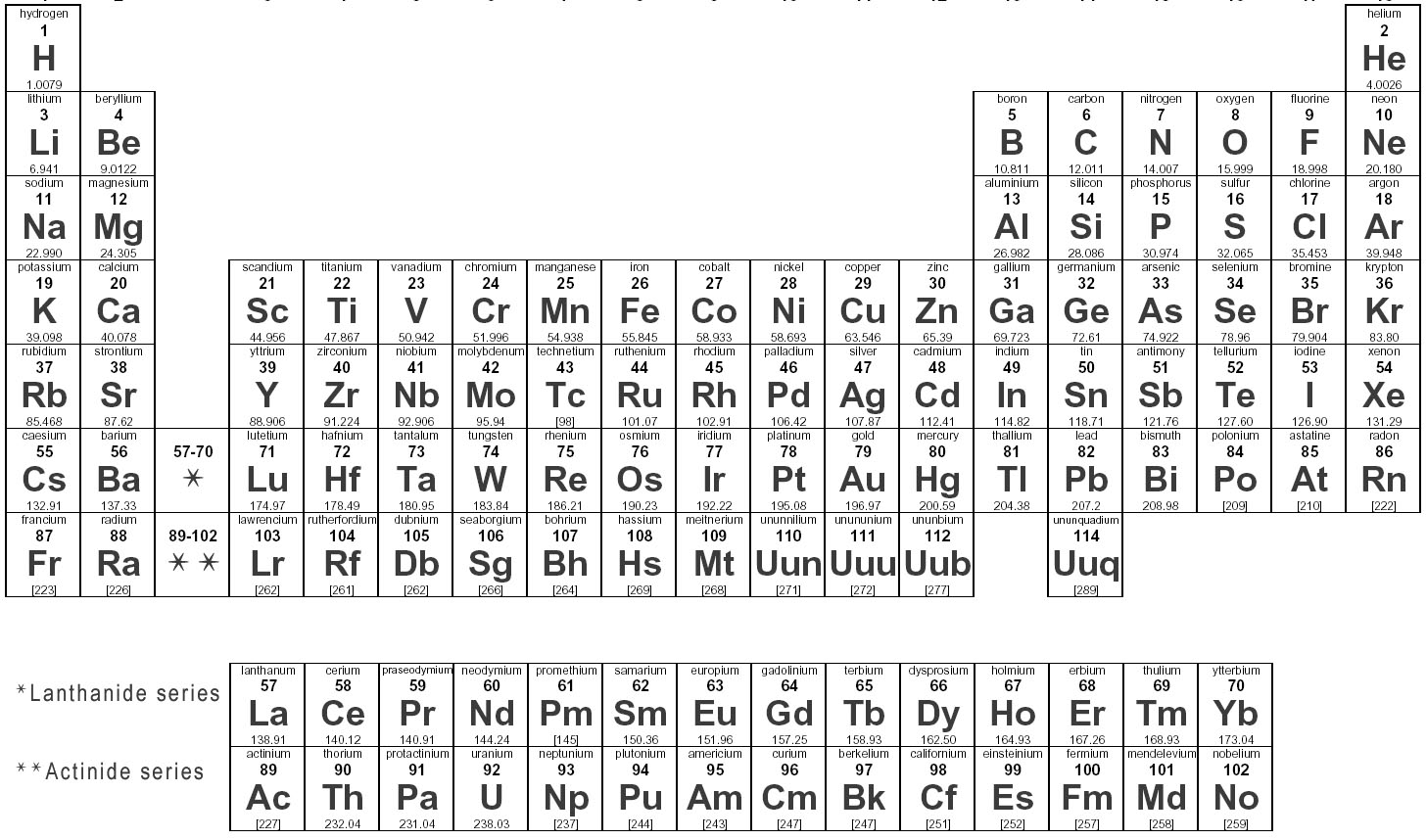
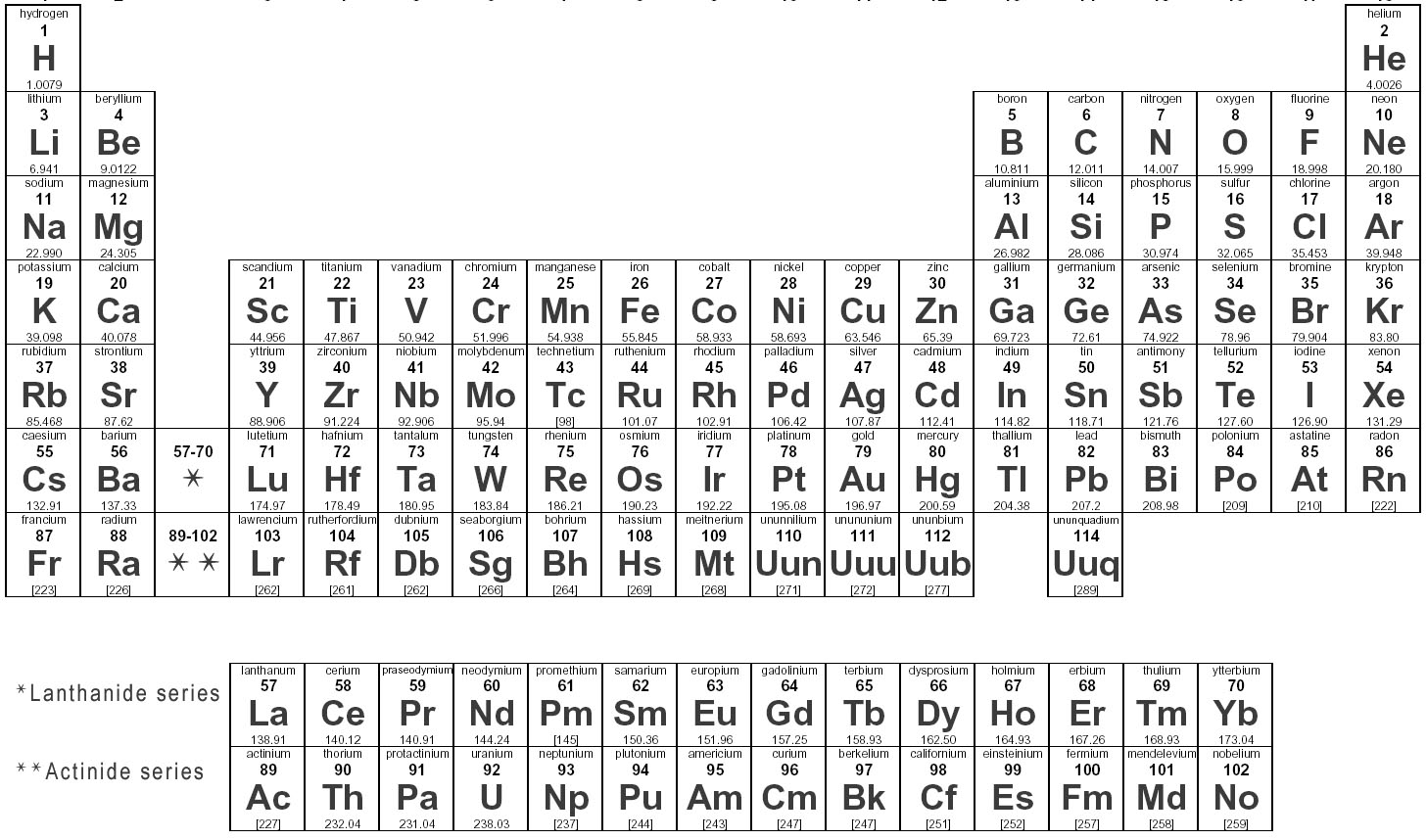
What are elements?
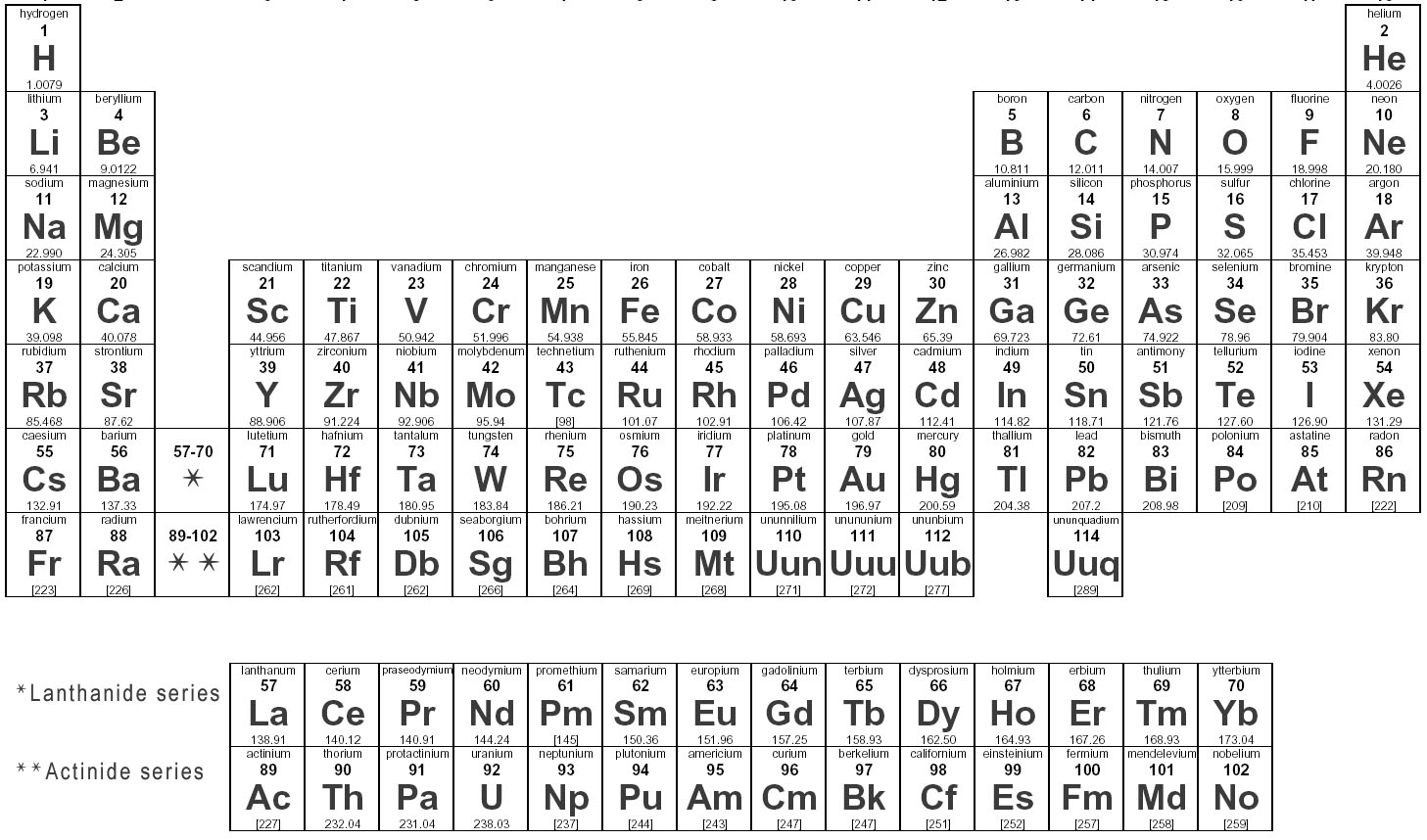
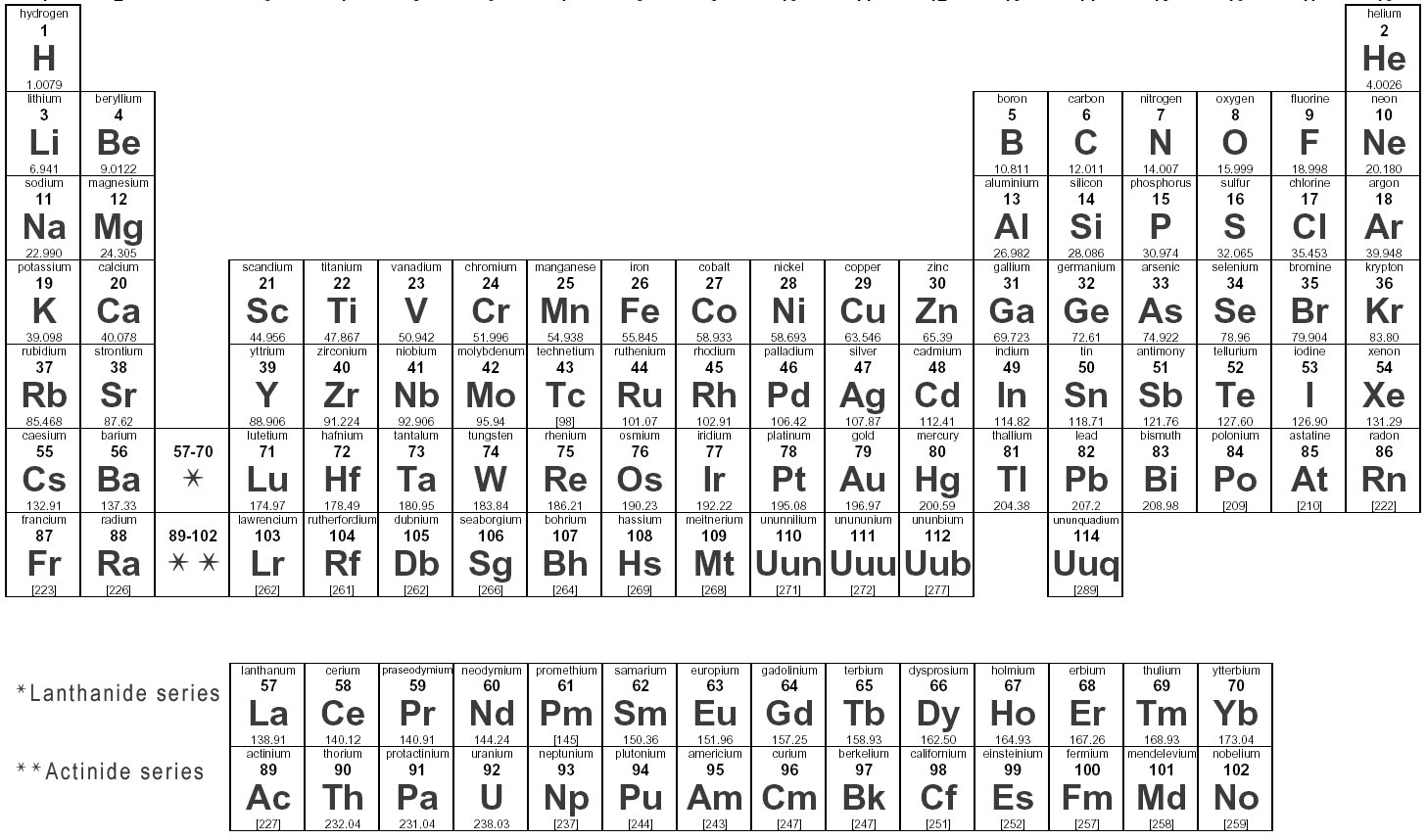
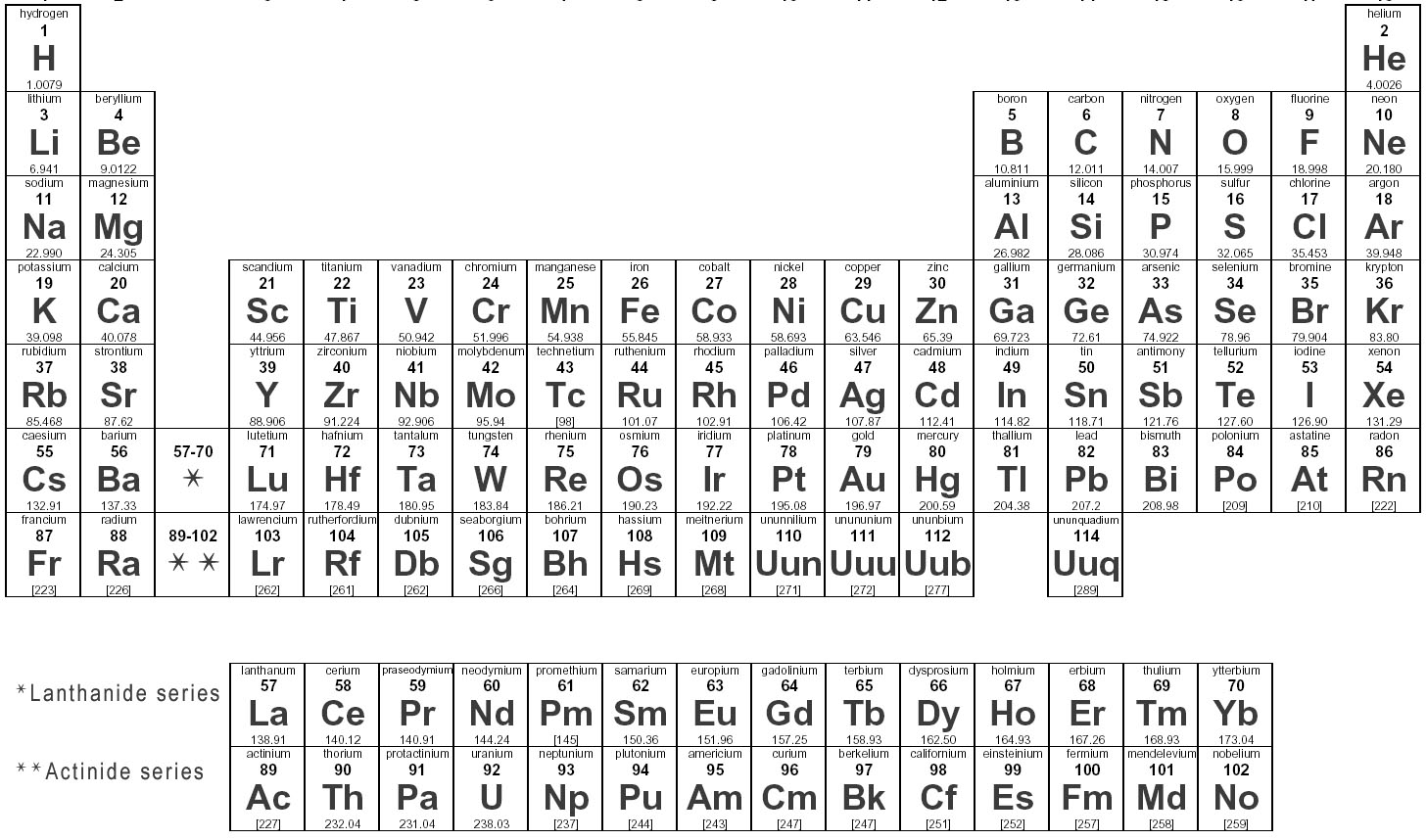
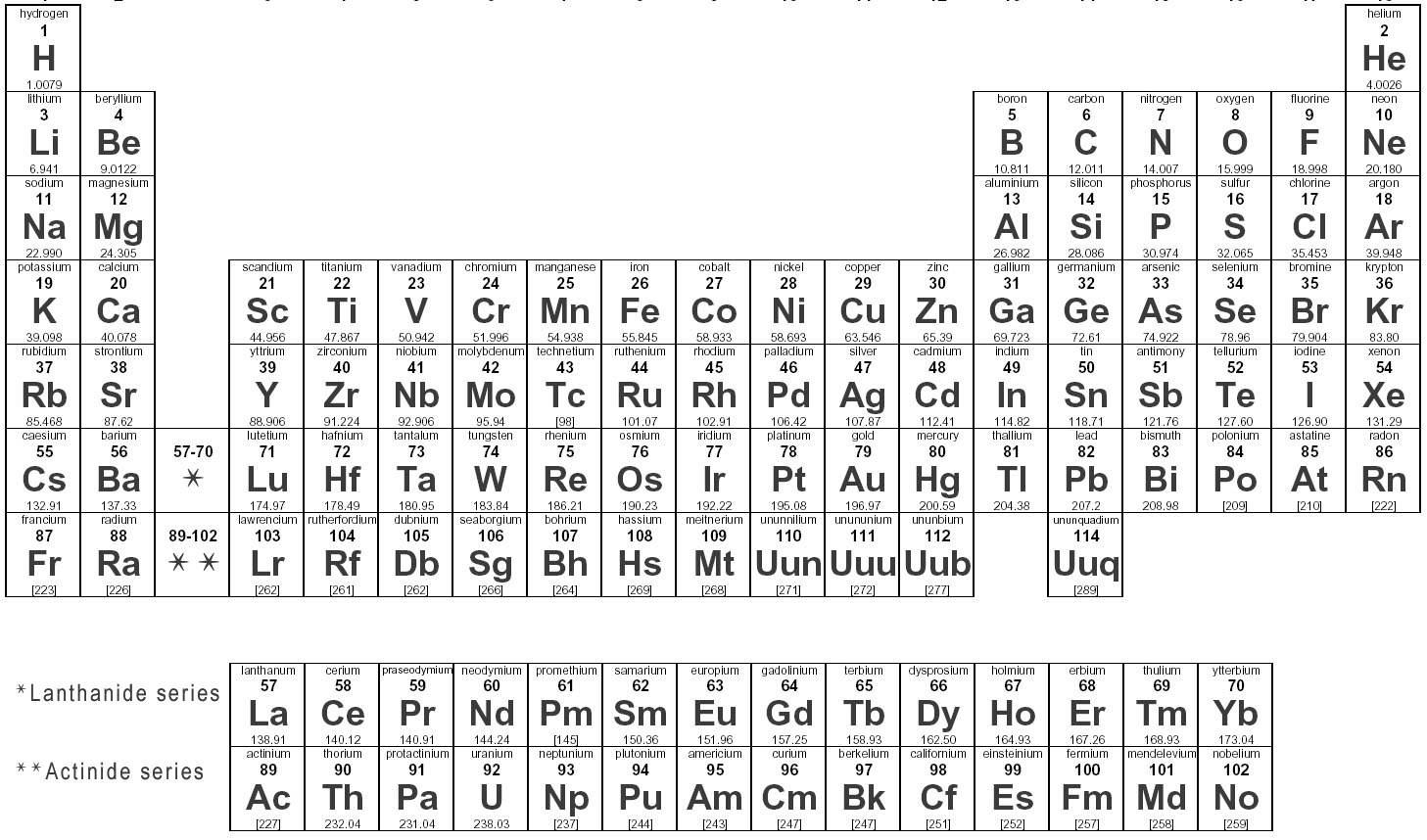
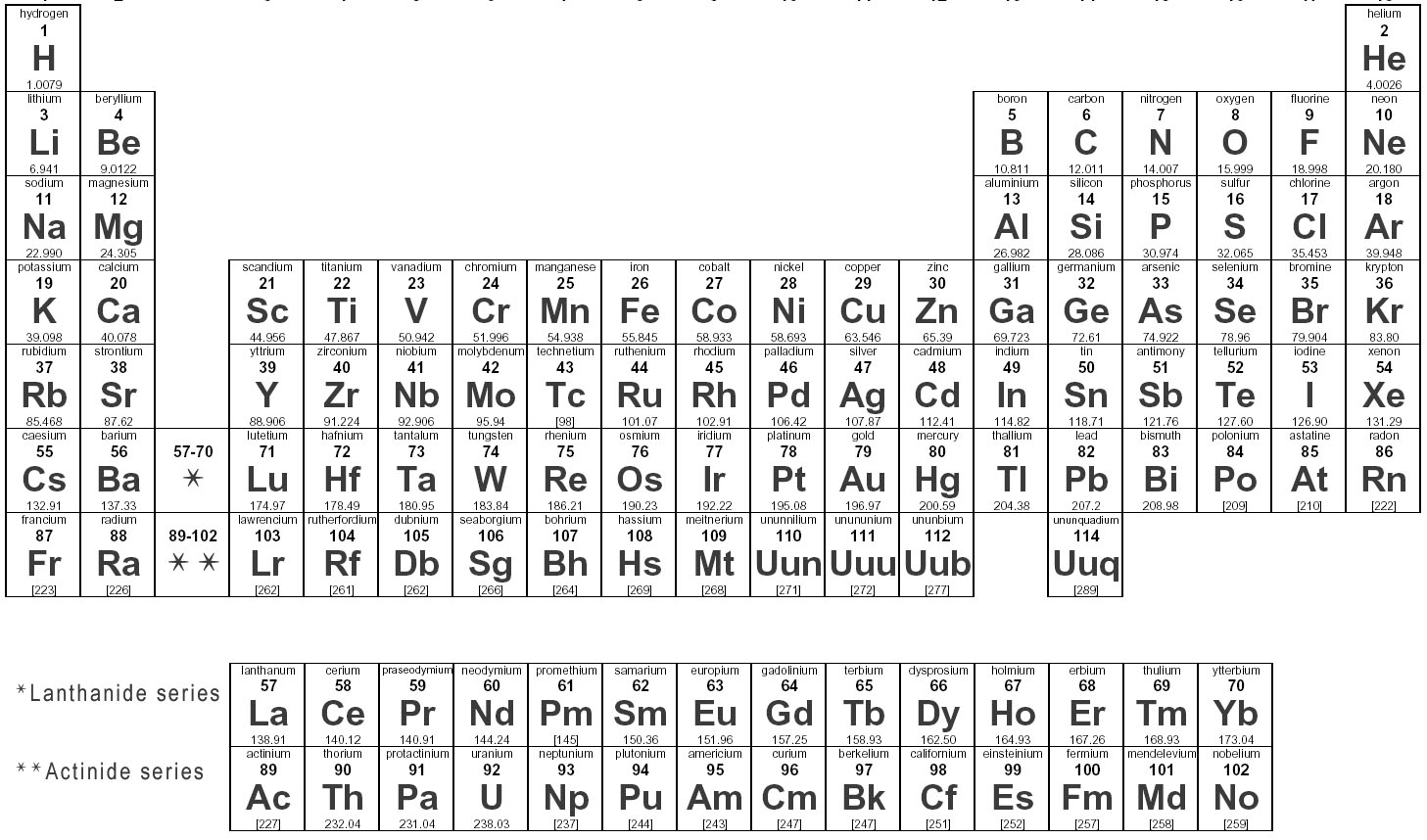
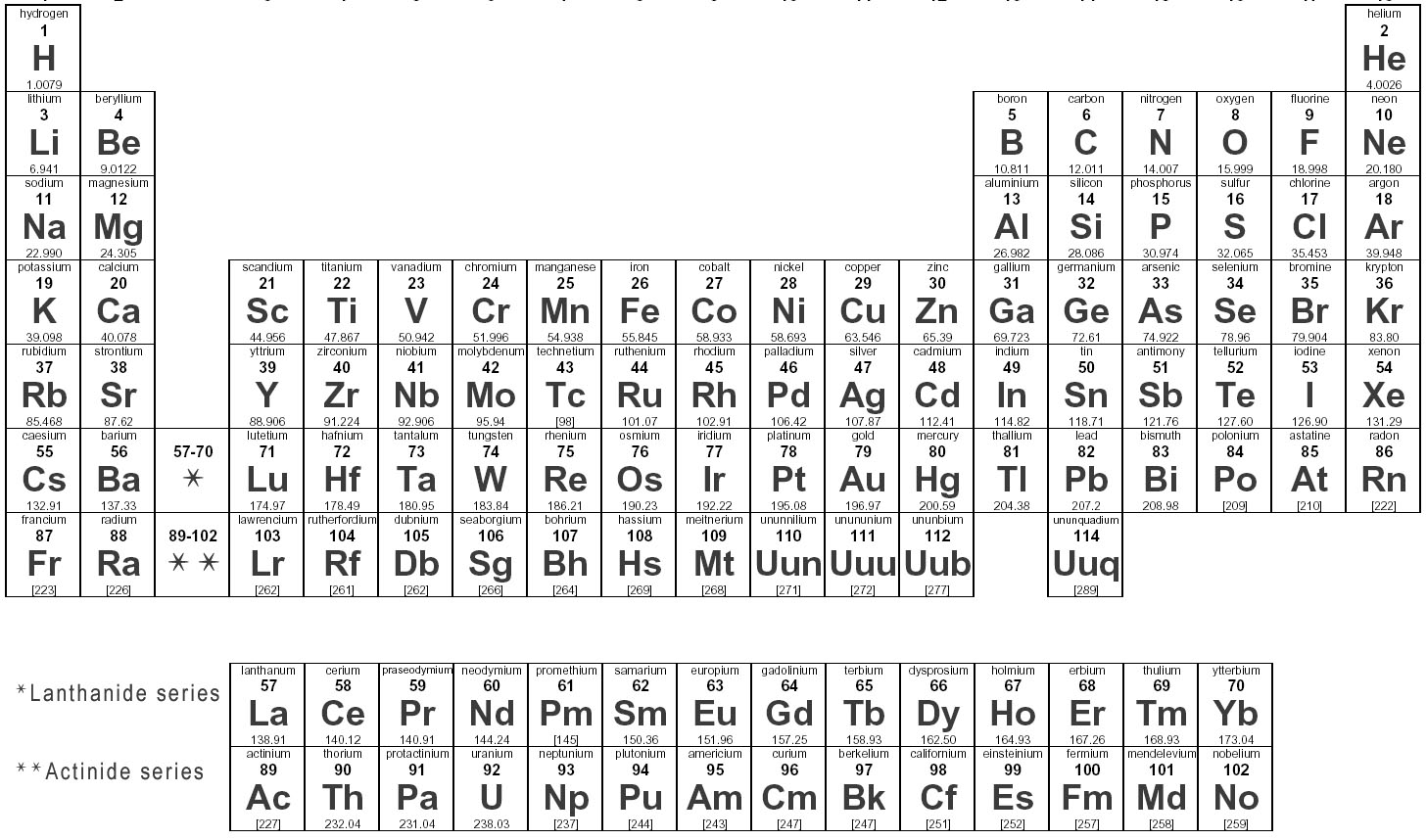
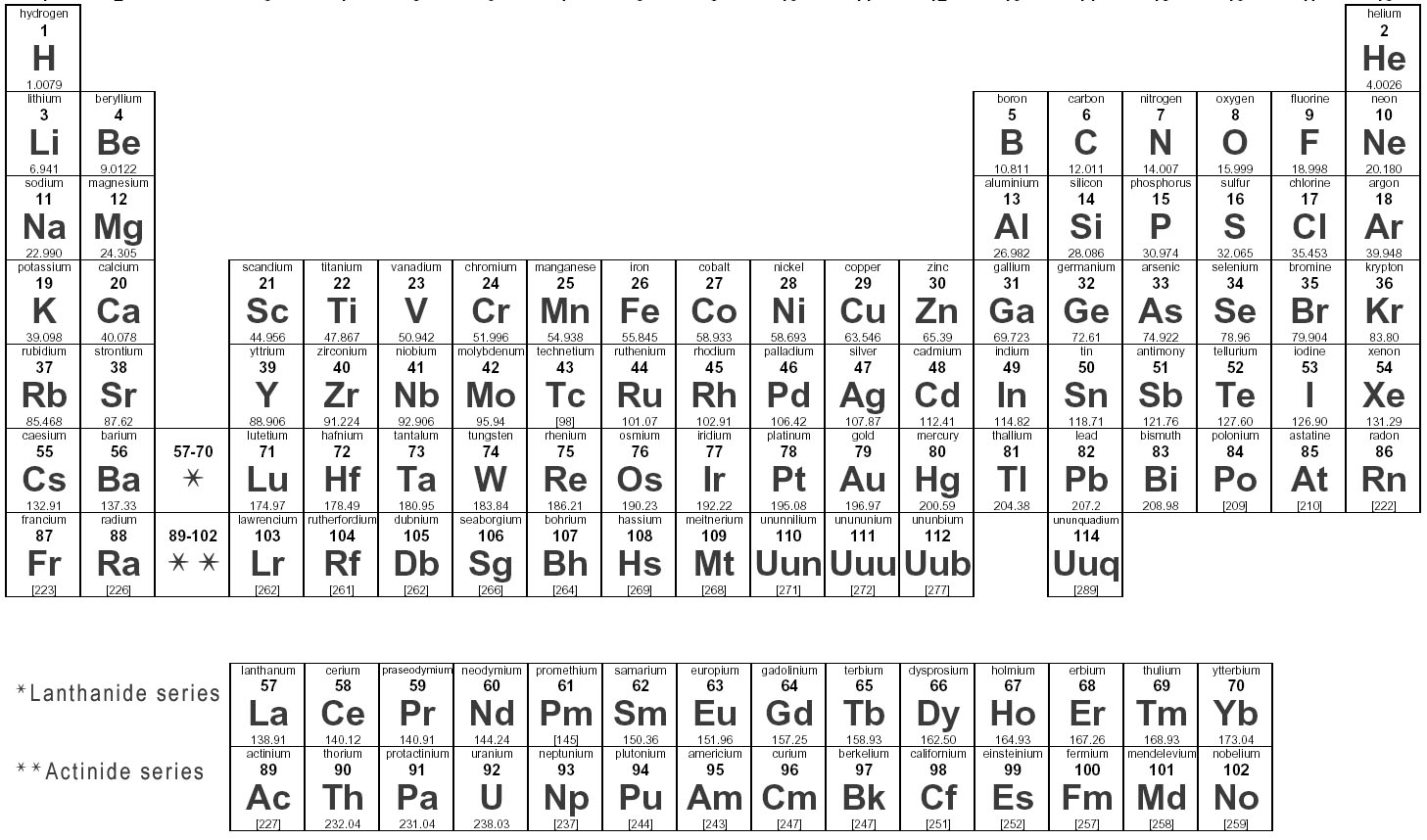
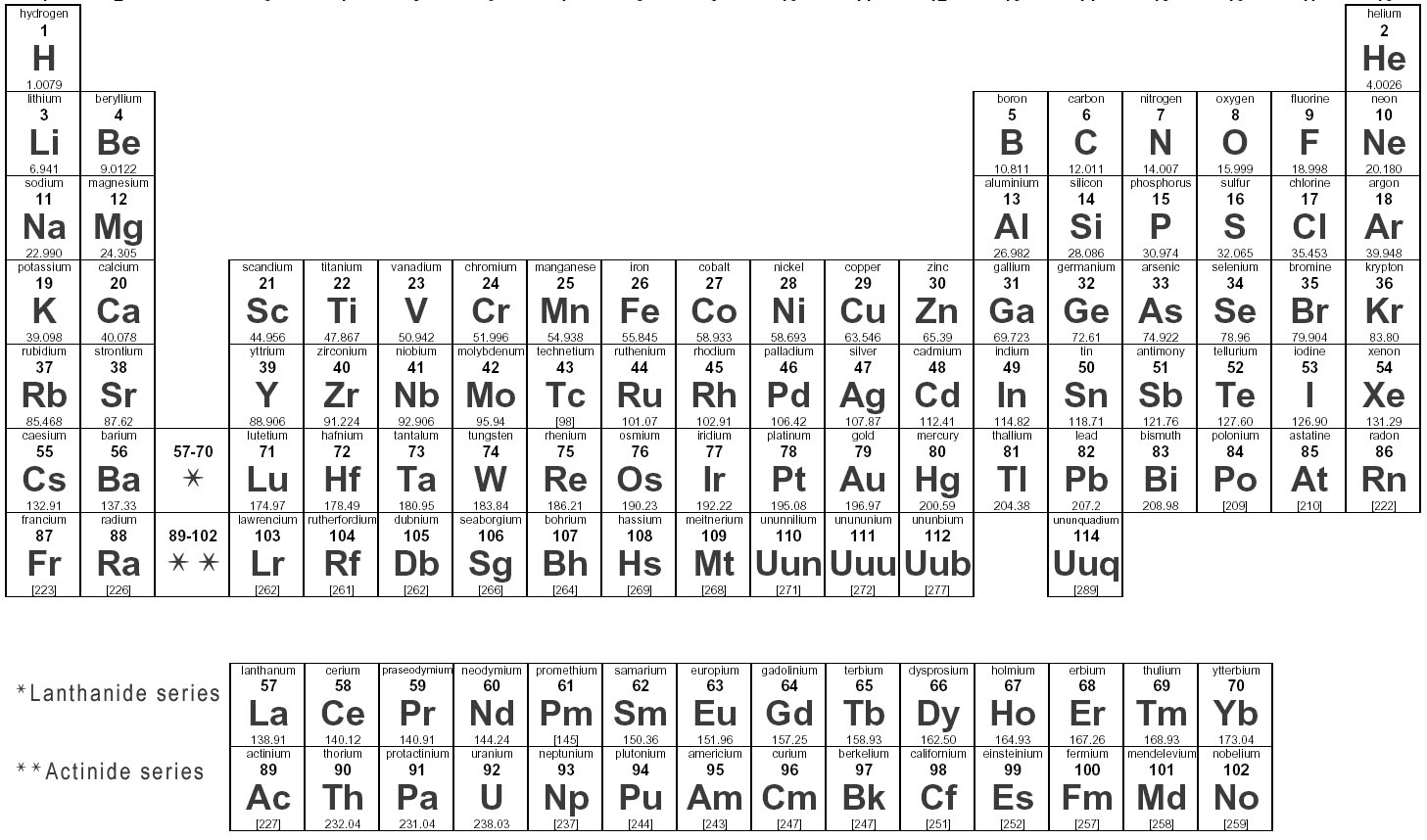
Elements are substances that are made up of only one type of atom, which is the smallest unit of matter. They are organized in the periodic table based on their atomic number, which represents the number of protons in their nucleus. Elements can combine to form compounds, and they play a fundamental role in the composition of all matter in the universe.
What is the difference between a compound and an element?
An element is a substance that is made up of only one type of atom, with distinct chemical properties, represented by its unique symbol on the periodic table. On the other hand, a compound is a substance made up of two or more different elements that are chemically bonded together in fixed proportions, resulting in a new substance with different properties than the individual elements. Essentially, elements are the building blocks of compounds, which are formed through chemical reactions.
How are compounds formed?
Compounds are formed when two or more elements chemically combine through bonding to create a new substance with unique properties. This bonding can occur through various types of chemical reactions, such as ionic bonding (transfer of electrons), covalent bonding (sharing of electrons), or metallic bonding (delocalized electrons). The resulting compound has a distinct chemical composition and structure that differs from the properties of its individual elements.
What are some common examples of compounds?
Common examples of compounds include water (H2O), carbon dioxide (CO2), table salt (NaCl), glucose (C6H12O6), methane (CH4), ammonia (NH3), and ethanol (C2H5OH). These compounds are formed when two or more different elements chemically combine in specific ratios to create a new substance with unique properties.
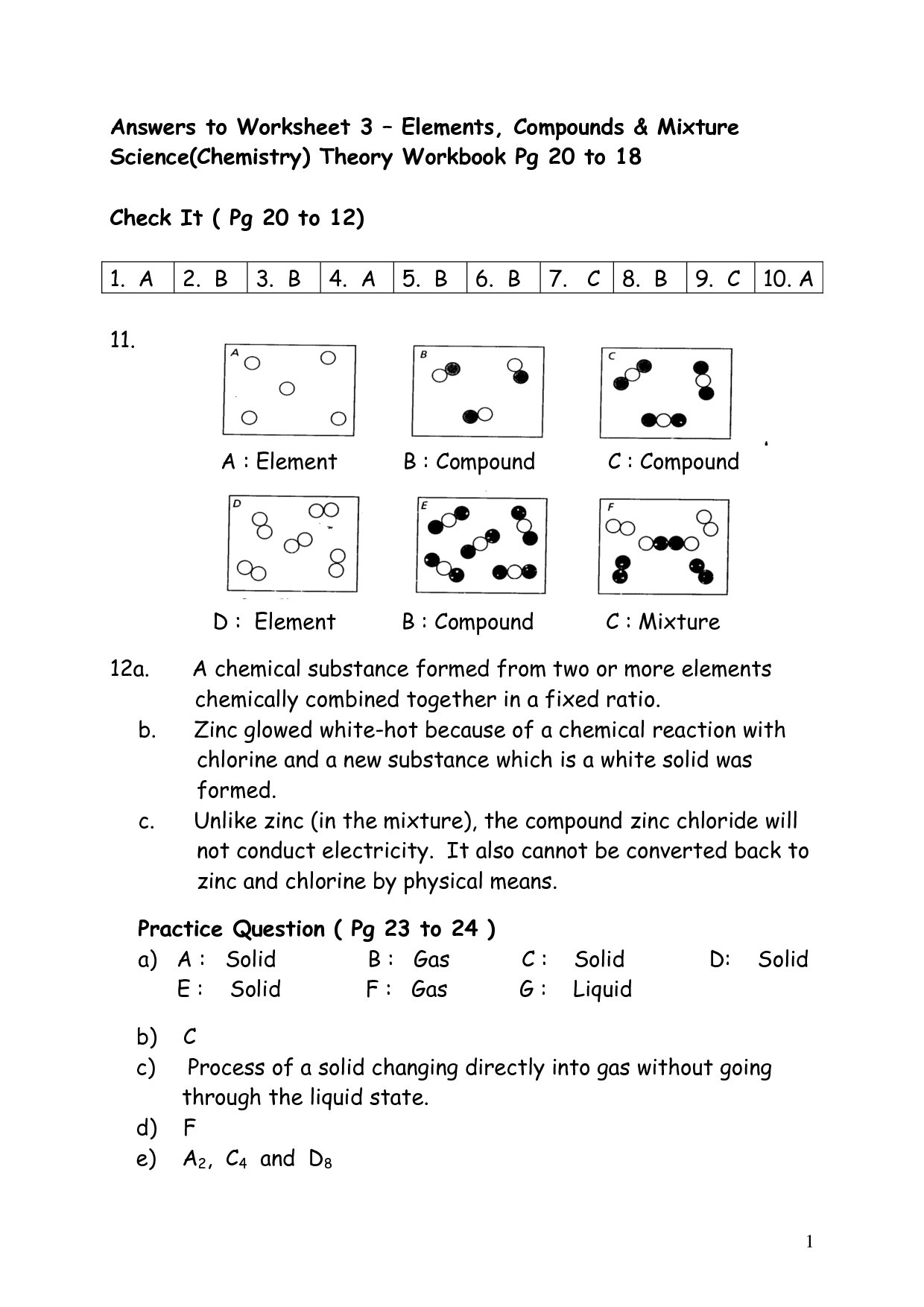
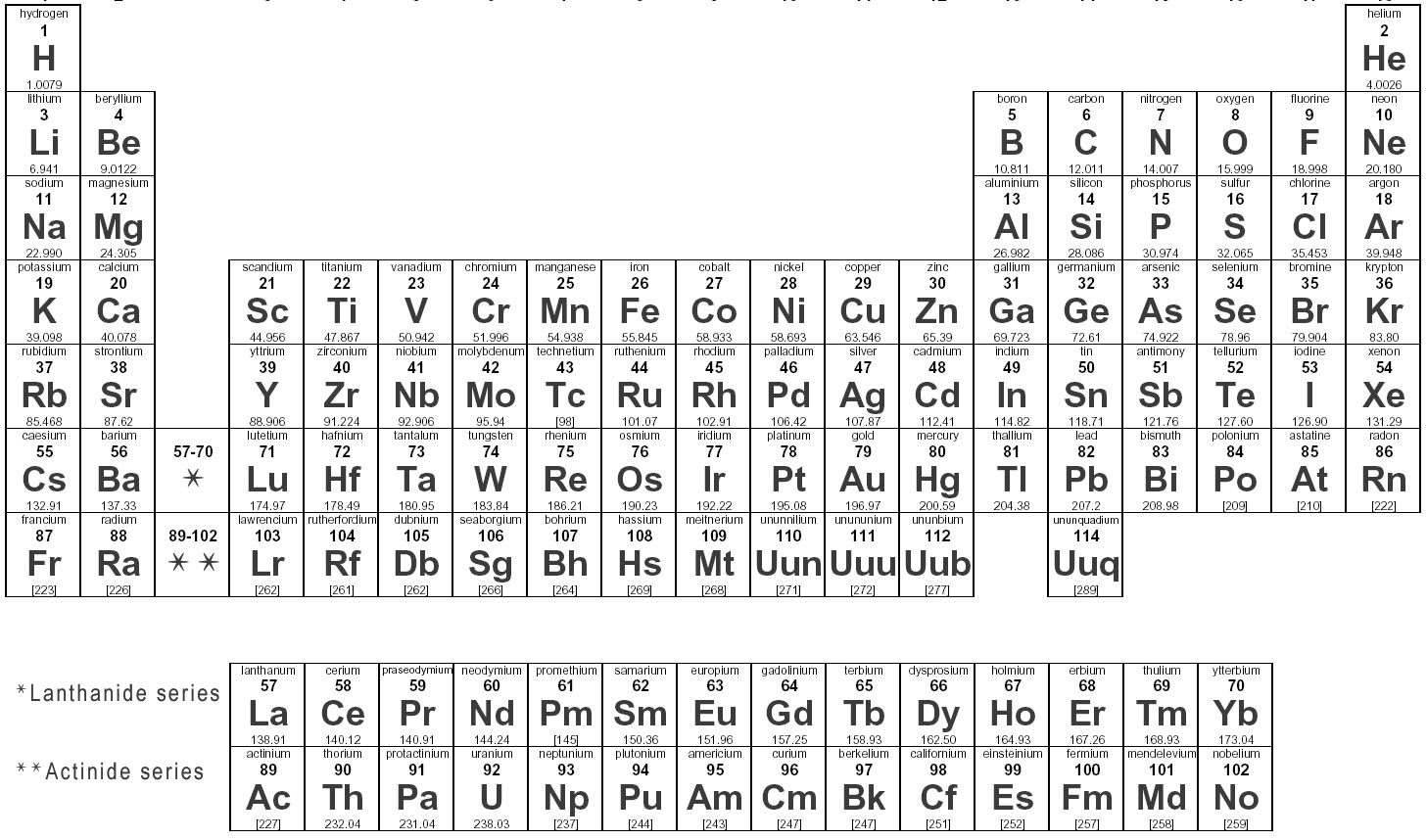
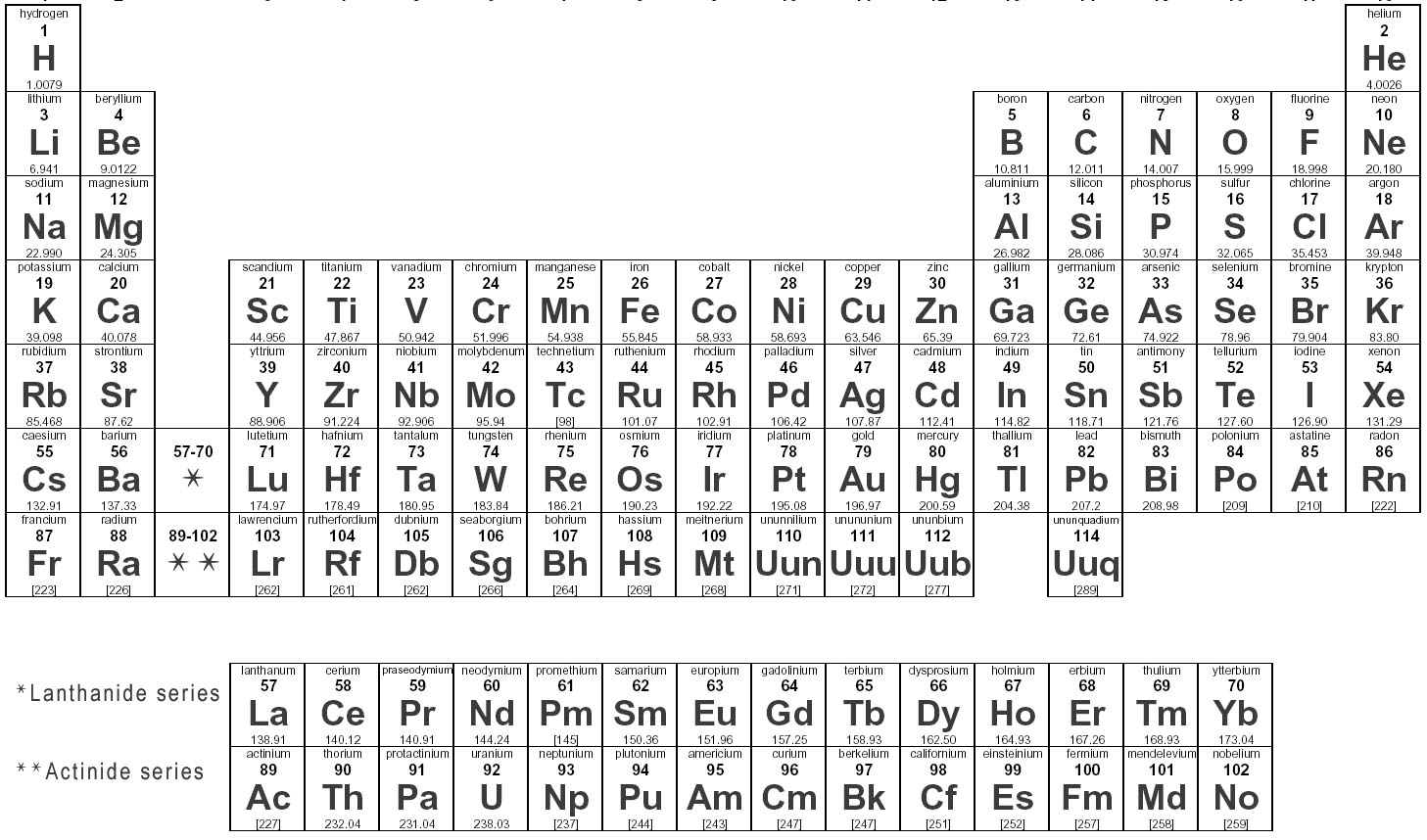
How are elements represented in the periodic table?
Elements are represented in the periodic table based on their atomic number, which is the number of protons in their nucleus. They are arranged in rows and columns based on similar chemical properties and increasing atomic number, creating a systematic way to classify and organize the elements. The periodic table also displays information about the elements' atomic mass, symbol, and electron configuration.
Can elements be broken down into simpler substances?
Yes, elements can be broken down into simpler substances. Elements are made up of atoms, which are the basic building blocks of matter. Atoms can combine with each other through chemical reactions to form compounds, which are made up of different elements. Therefore, elements can be broken down into simpler substances through chemical reactions and processes such as electrolysis or nuclear reactions.
How do compounds differ from mixtures?
Compounds are substances formed by the chemical combination of two or more elements in fixed proportions, whereas mixtures are combinations of different substances that are physically mixed together and can be separated by physical means. Compounds have specific chemical properties distinct from their individual elements, whereas mixtures retain the properties of their components.
How can compounds be identified and classified?
Compounds can be identified and classified through various analytical techniques such as spectroscopy, mass spectrometry, and chromatography, which help determine their chemical properties and structural characteristics. Identification is based on comparing experimental data to known standards, while classification is done based on the compound's chemical composition, functional groups, and physical properties. Ultimately, compounds are classified into groups such as organic and inorganic compounds, as well as further subcategories based on their specific properties and structures.
How are elements and compounds used in everyday life?
Elements and compounds are used in everyday life in various ways. For example, oxygen, a vital element, is used by our bodies for respiration. Water, a compound made up of hydrogen and oxygen, is essential for hydration. Common table salt, a compound made of sodium and chlorine, is used for seasoning food. Additionally, compounds like plastics, detergents, medications, and fuels are all examples of how elements and compounds play crucial roles in our daily activities, from household chores to transportation and healthcare.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.






















Comments