Types of Chemical Bonds Worksheet Answers
Chemical bonds are a fundamental concept in chemistry, and understanding the different types of bonds is essential for success in the subject. If you are a chemistry student or someone who wants to explore the world of chemical bonding, you may be seeking suitable resources to enhance your understanding. In this blog post, we will provide you with a comprehensive list of worksheets that offer answers to various types of chemical bonds.
Table of Images 👆
- Chemical Bonding Worksheet Answer Key
- Balancing Chemical Equations Worksheet Answer Key
- Ionic and Covalent Bonding Worksheet
- Chemical Bonding Worksheet Answers
- Lewis Dot Covalent Bond Worksheet
- Come Together Chemical Bonding Worksheet
- Compounds Covalent Bonds Worksheet Answers
- Chemistry Chemical Bonding Worksheet Answers Activity
- Naming Ionic Compounds Worksheet Answer Key
- Types of Chemical Bonds Worksheet Answer Key
- Naming Ionic Compounds Worksheet Answers
- Writing Ionic Compound Formula Worksheet Answers
- Practice Ionic Covalent Compound Worksheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
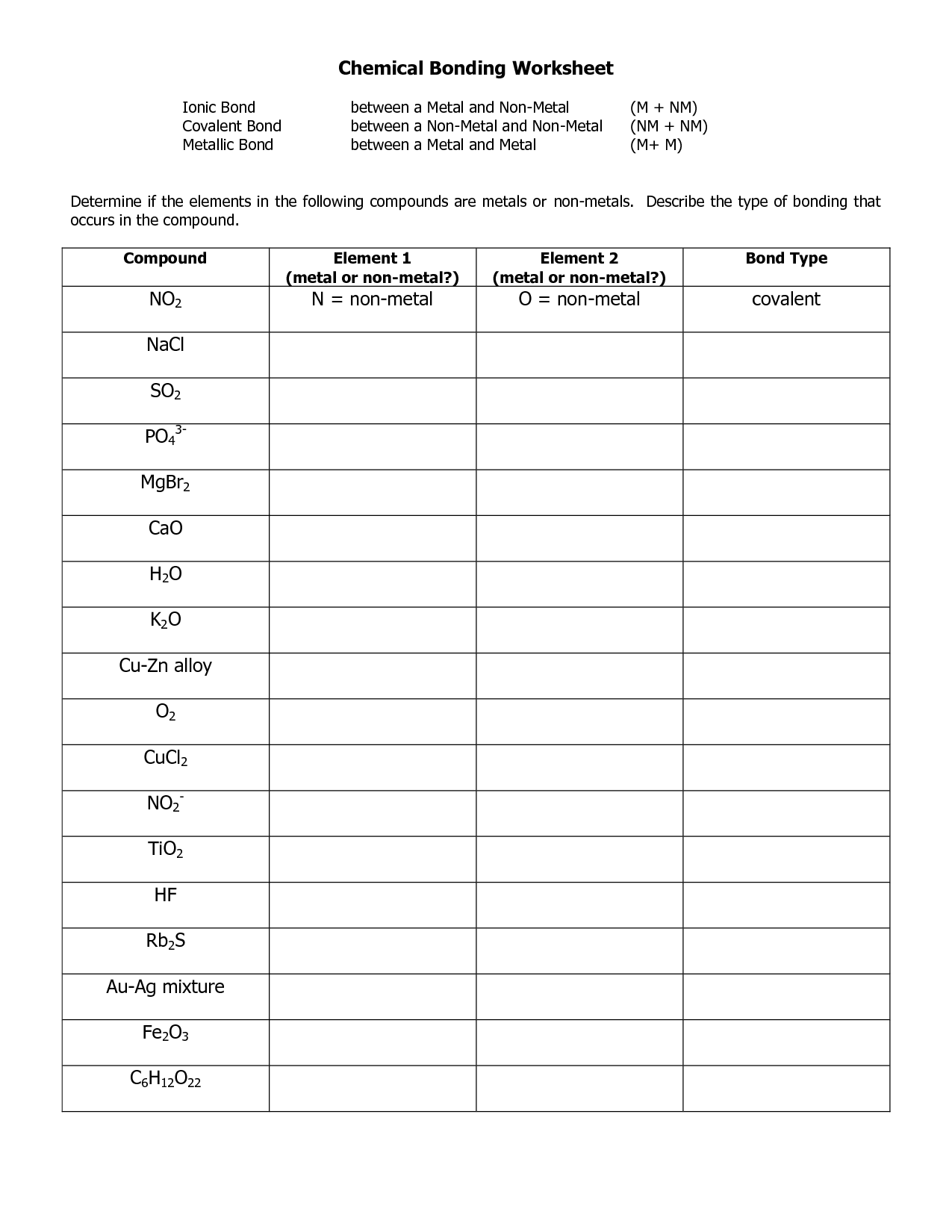
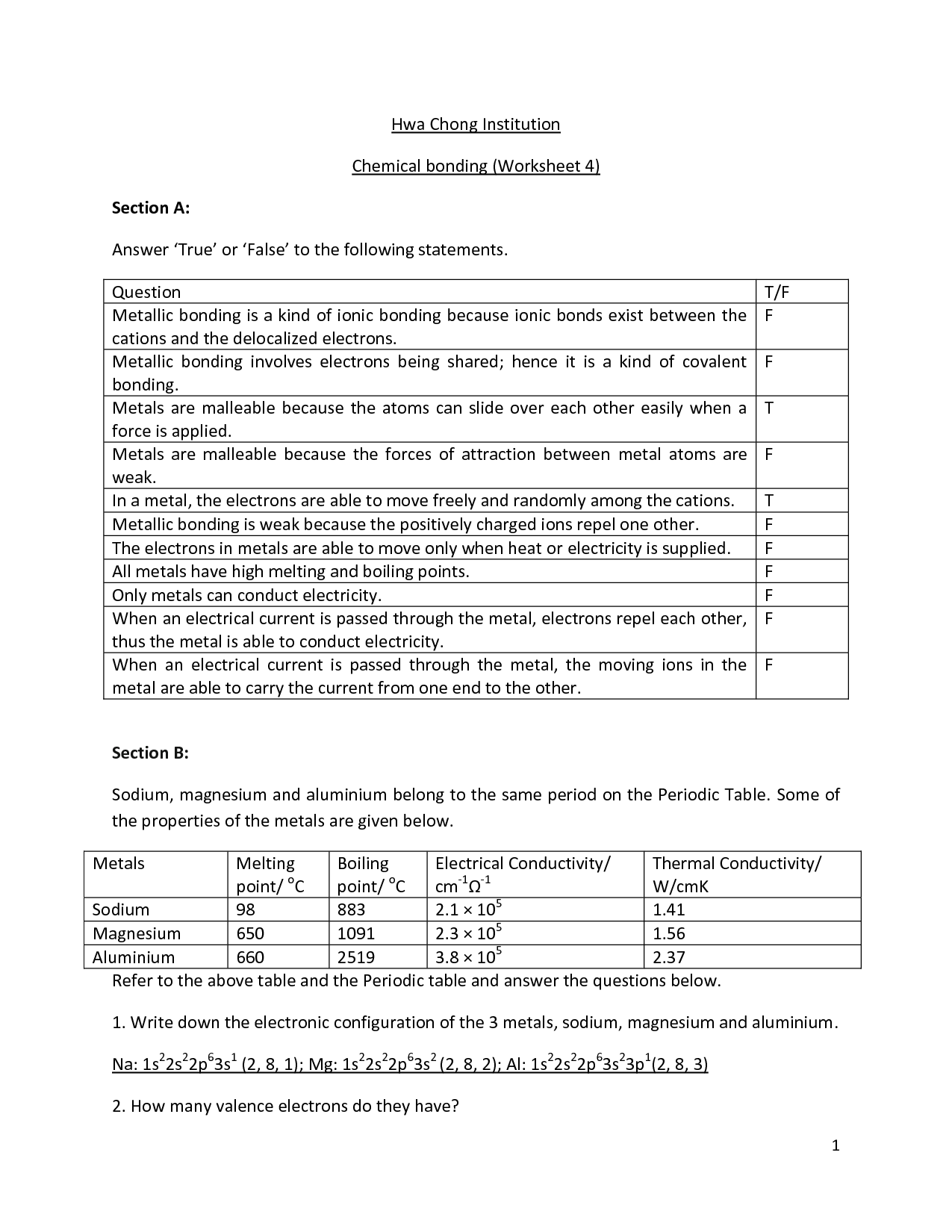
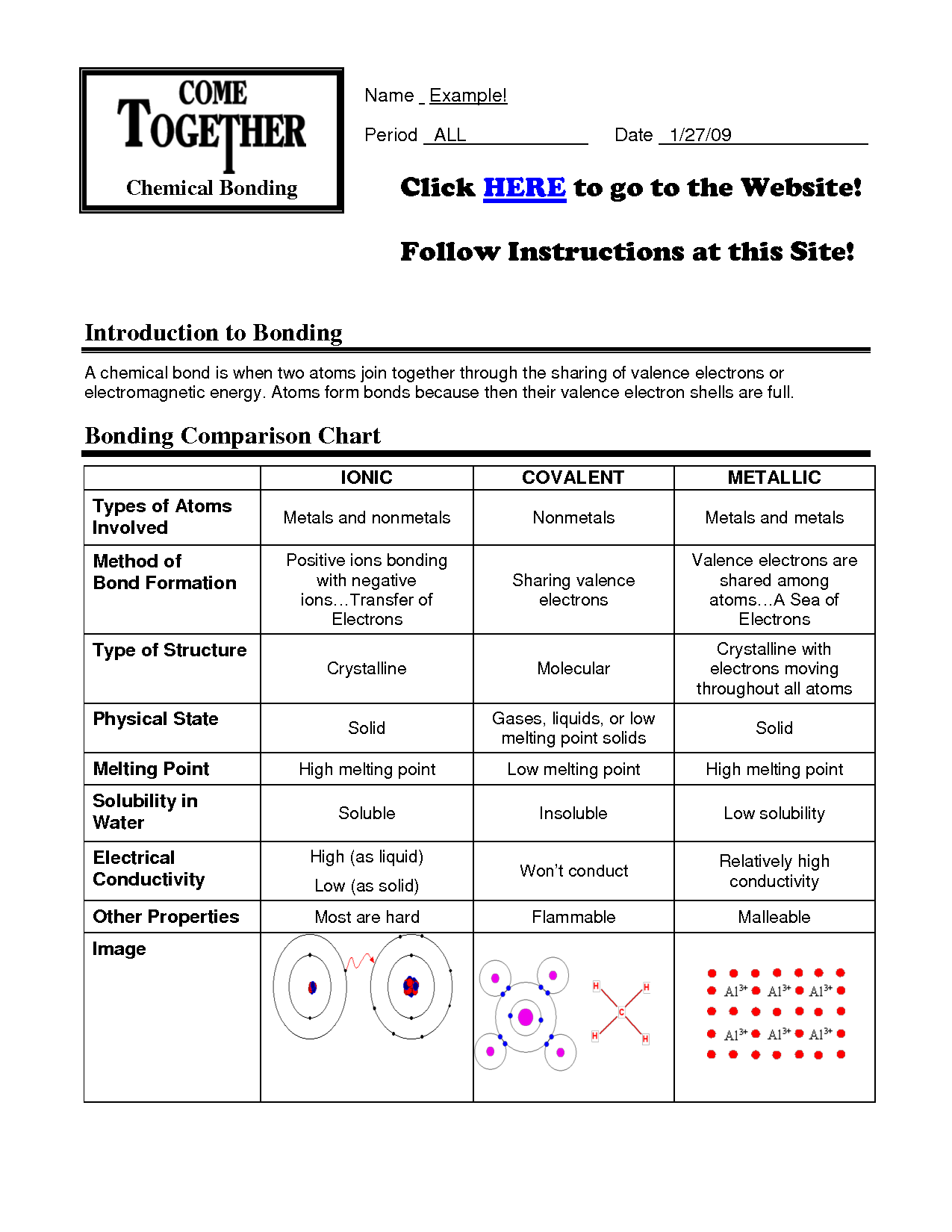
Ionic bonds occur when there is a complete transfer of electrons from one atom to another.
Ionic bonds form when one atom donates an electron(s) to another atom, resulting in the creation of positively and negatively charged ions that are attracted to each other electrostatically. This transfer of electrons leads to the formation of a strong bond between the two atoms due to the attraction of opposite charges.
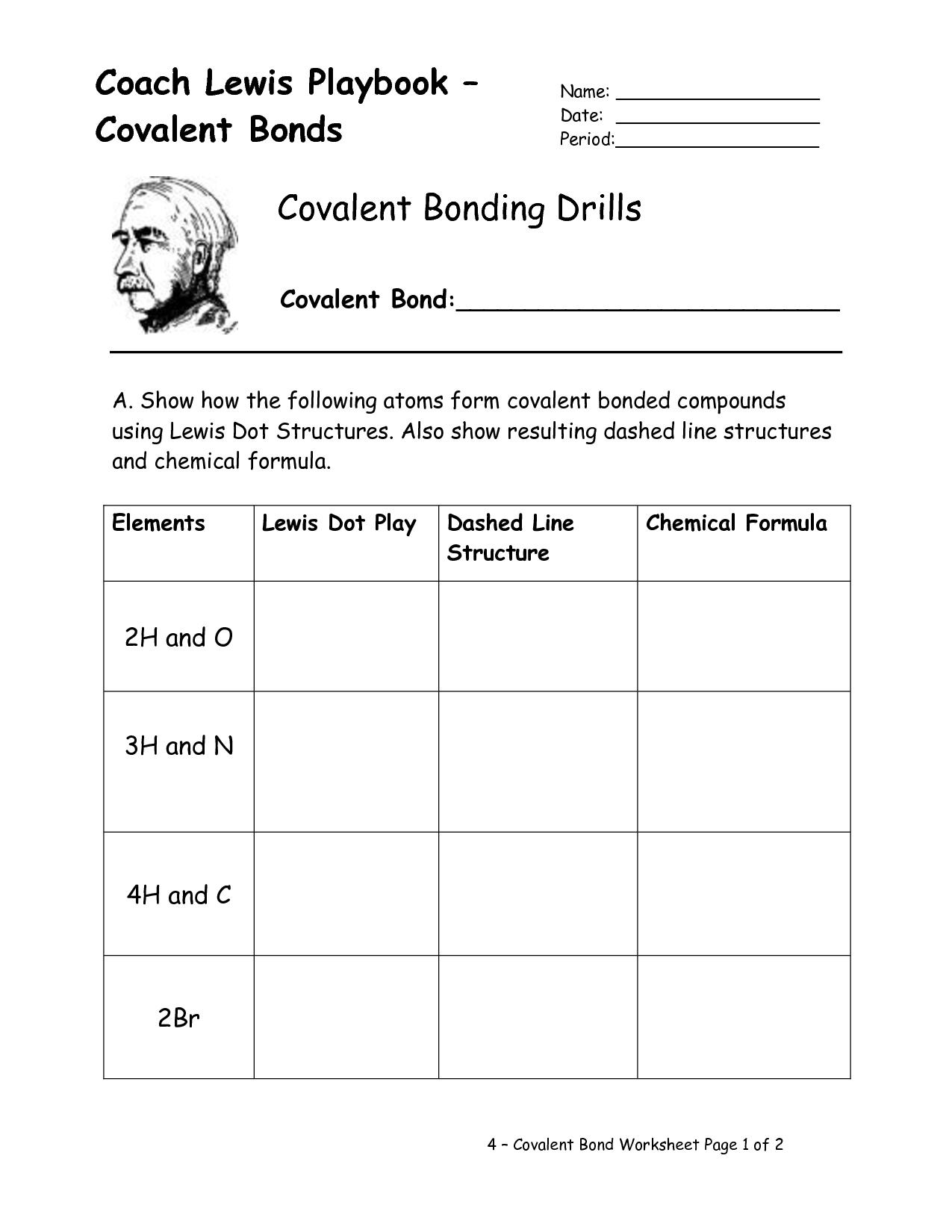
Covalent bonds involve the sharing of electrons between atoms to achieve a stable electron configuration.
Correct, covalent bonds are formed when two atoms share a pair of electrons in order to fill their outer electron shells and become more stable. This sharing allows atoms to achieve a full valence shell, similar to the noble gases, and results in the formation of molecules.
Metallic bonds occur between metal atoms and involve the sharing of a "sea" of valence electrons.
Metallic bonds occur between metal atoms as the atoms release their valence electrons to form a "sea" of delocalized electrons that are free to move throughout the structure. This sharing of electrons creates a stable network of positive metal ions surrounded by a cloud of negative electrons, leading to the unique properties of metals such as malleability, ductility, and excellent thermal and electrical conductivity.
Polar covalent bonds occur when the electrons in a covalent bond are unequally shared, resulting in partial positive and negative charges.
Yes, that is correct. In polar covalent bonds, the electrons are not shared equally between the atoms, leading to an uneven distribution of electrons and partial charges. This results in one atom having a slight positive charge and the other a slight negative charge, creating a polar molecule.
Nonpolar covalent bonds occur when the electrons in a covalent bond are equally shared, resulting in no partial charges.
Nonpolar covalent bonds form when two atoms share electrons equally, leading to a balanced distribution of charge and no separation of charges within the bond. This equilibrium in electron sharing allows for a stable, harmonious bond where the electrons are evenly distributed between the bonded atoms.
Hydrogen bonds occur when a hydrogen atom that is covalently bonded to an electronegative atom is attracted to another electronegative atom.
Correct. Hydrogen bonds form between a hydrogen atom that is bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine, and another electronegative atom nearby. This attraction is due to the difference in electronegativity between the atoms involved, resulting in a relatively strong intermolecular force.
Van der Waals interactions are weak attractions between temporarily positive and negative regions of molecules due to random fluctuations in electron distributions.
Van der Waals interactions arise from the fluctuations in electron distributions within molecules, leading to weak attractions between temporarily positive and negative regions.
Dipole-dipole interactions occur between polar molecules, with the positive end of one molecule attracted to the negative end of another.
Correct. Dipole-dipole interactions are a type of intermolecular force that occurs between polar molecules due to the unequal distribution of electrons within the molecules, resulting in regions of partial positive and negative charges. This attraction between the positive and negative ends of adjacent molecules contributes to the overall stability and physical properties of the substance.
London dispersion forces are the weakest type of intermolecular force, resulting from temporary dipoles in nonpolar molecules.
London dispersion forces, also known as van der Waals forces, occur when electrons in a molecule are momentarily unevenly distributed, creating temporary dipoles that attract neighboring molecules. These forces are the weakest of all intermolecular forces and are commonly found in nonpolar molecules.
Network covalent bonds form in certain substances, such as diamond or quartz, where each atom is covalently bonded to its neighbors, forming a continuous network structure.
Network covalent bonds are formed in substances like diamond or quartz, where every atom is covalently bonded to its neighboring atoms, creating a continuous network structure that gives these materials their exceptional hardness and durability.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



























Comments