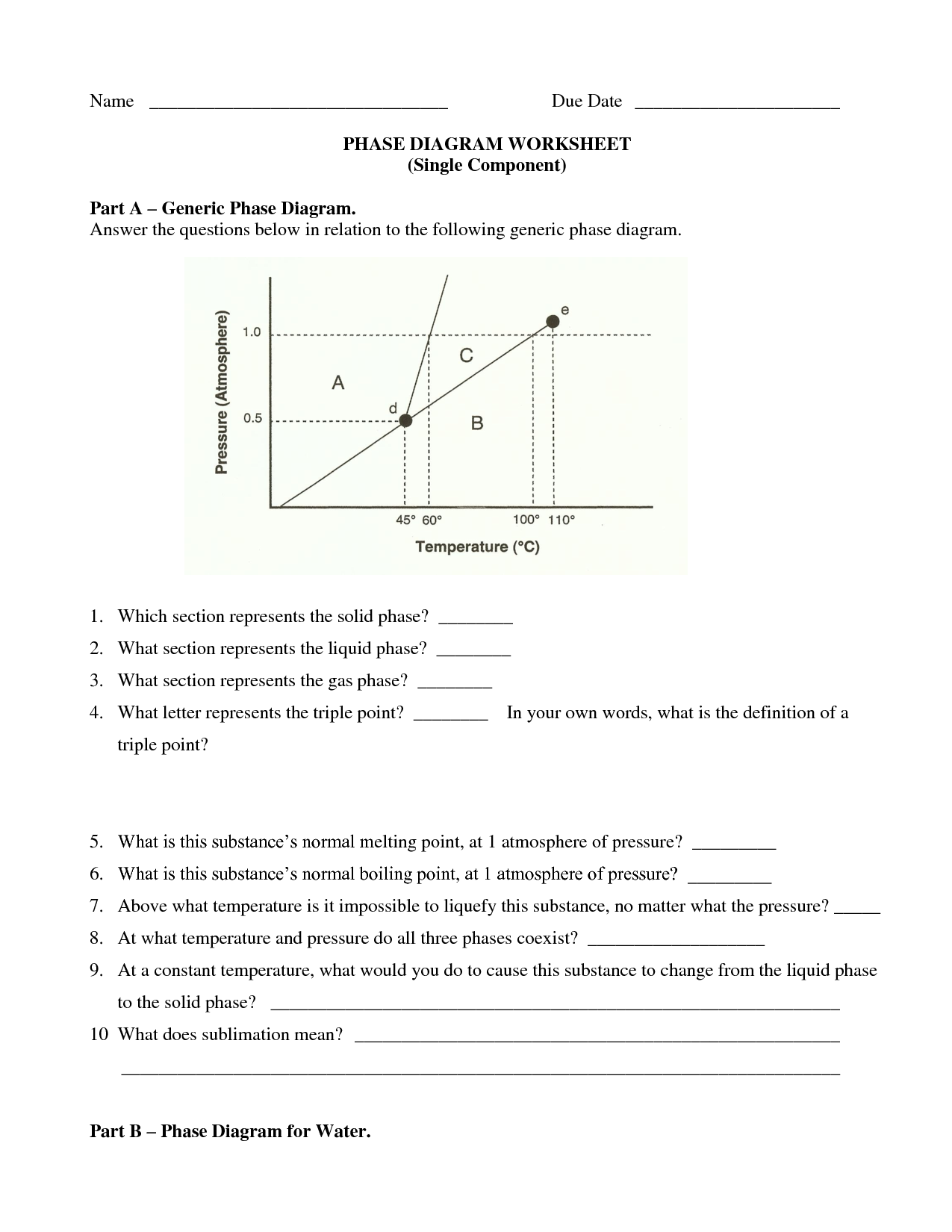
Phase Change Worksheet Answers
Are you a student or teacher seeking comprehensive answers to phase change worksheets? Look no further, as this blog post will provide you with the solutions you need. Whether you are studying chemistry, physics, or another science subject that covers phase changes, this post will delve into the entity of phase change and explore the corresponding worksheet answers.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is a phase change?
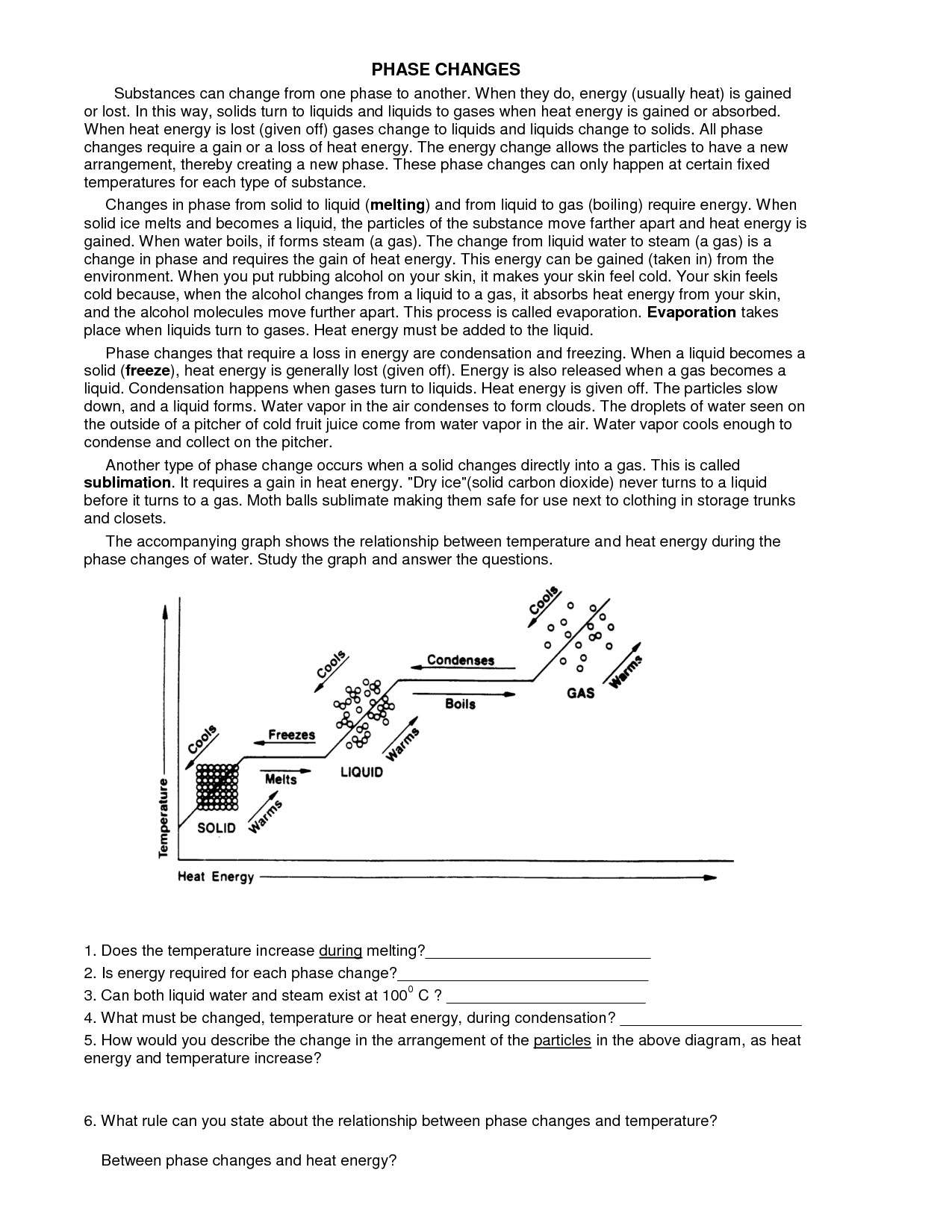
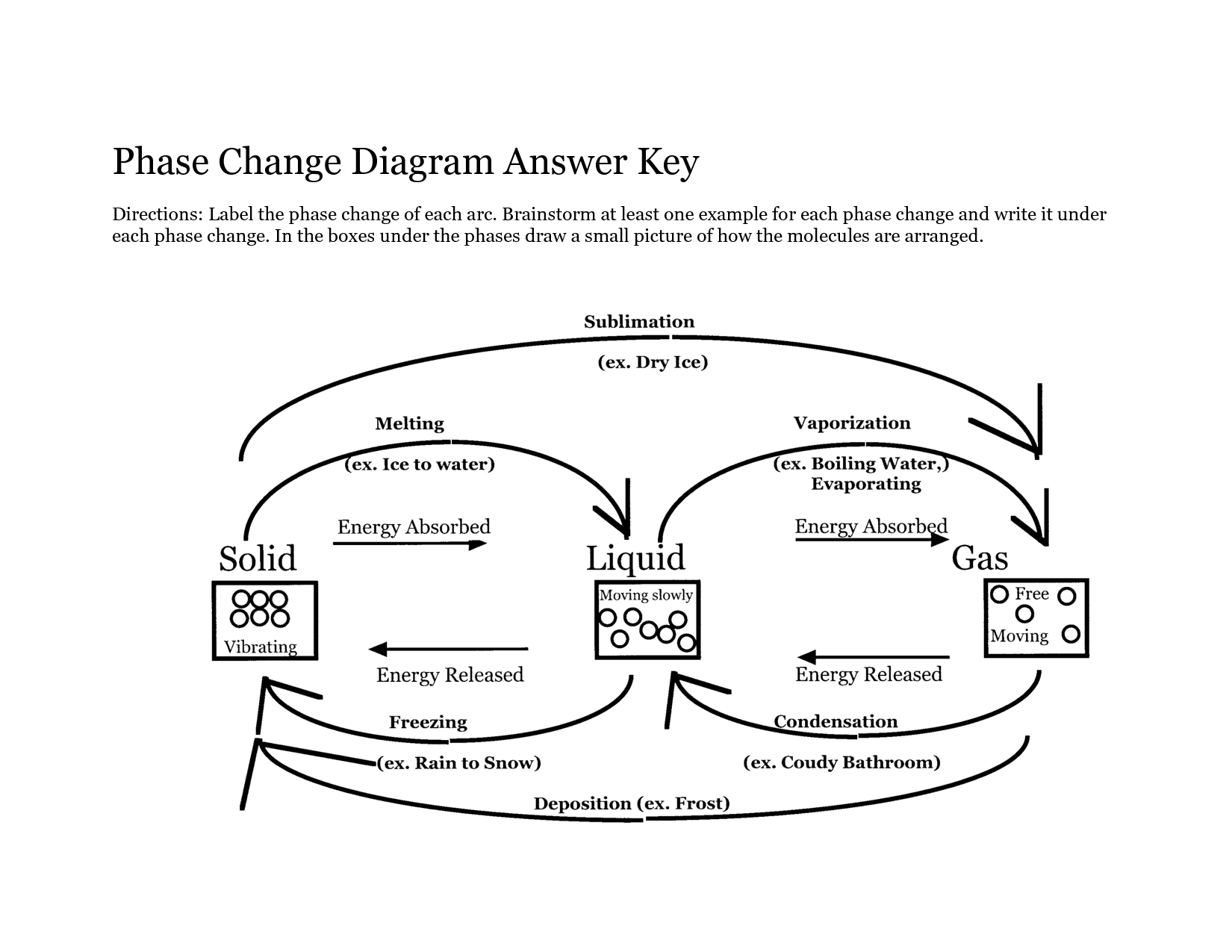
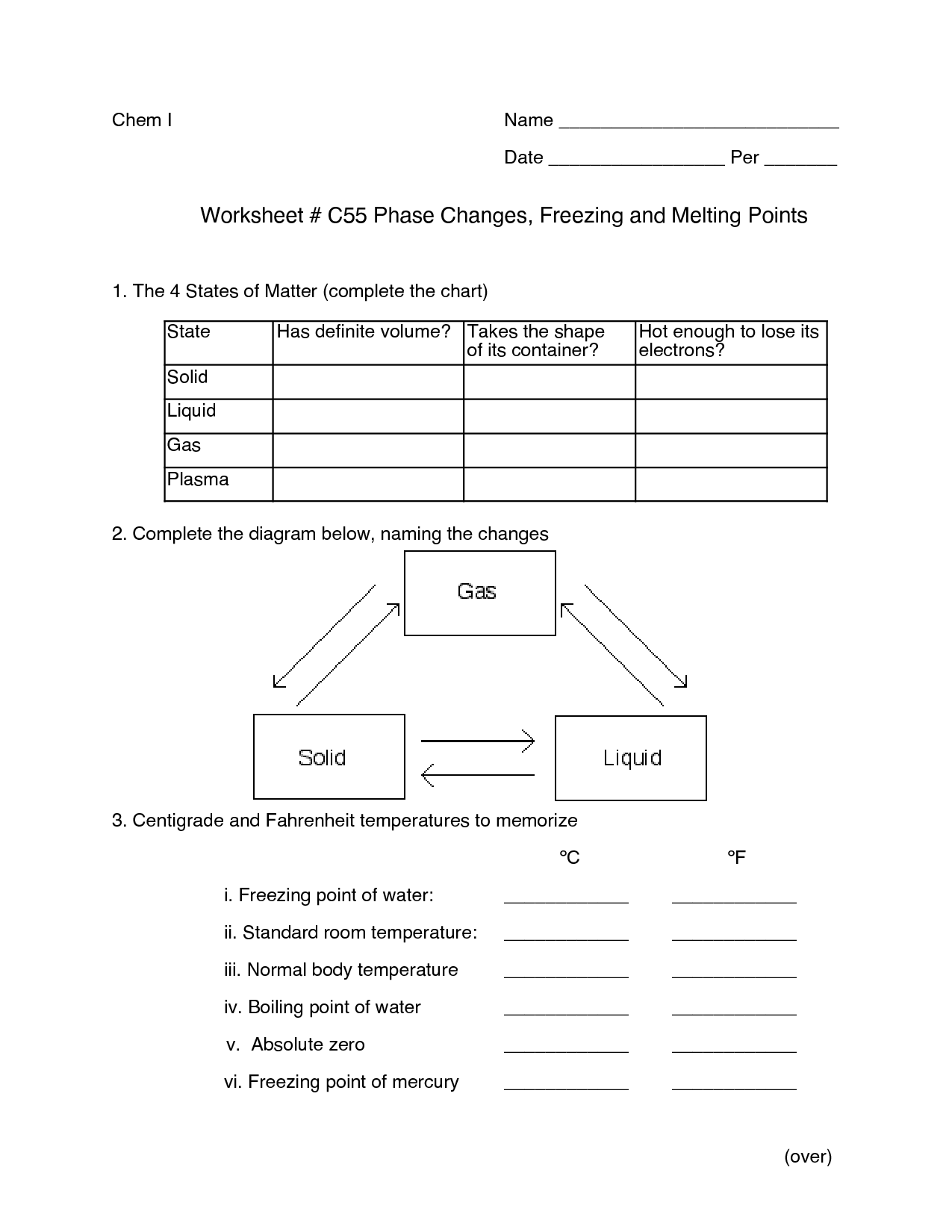
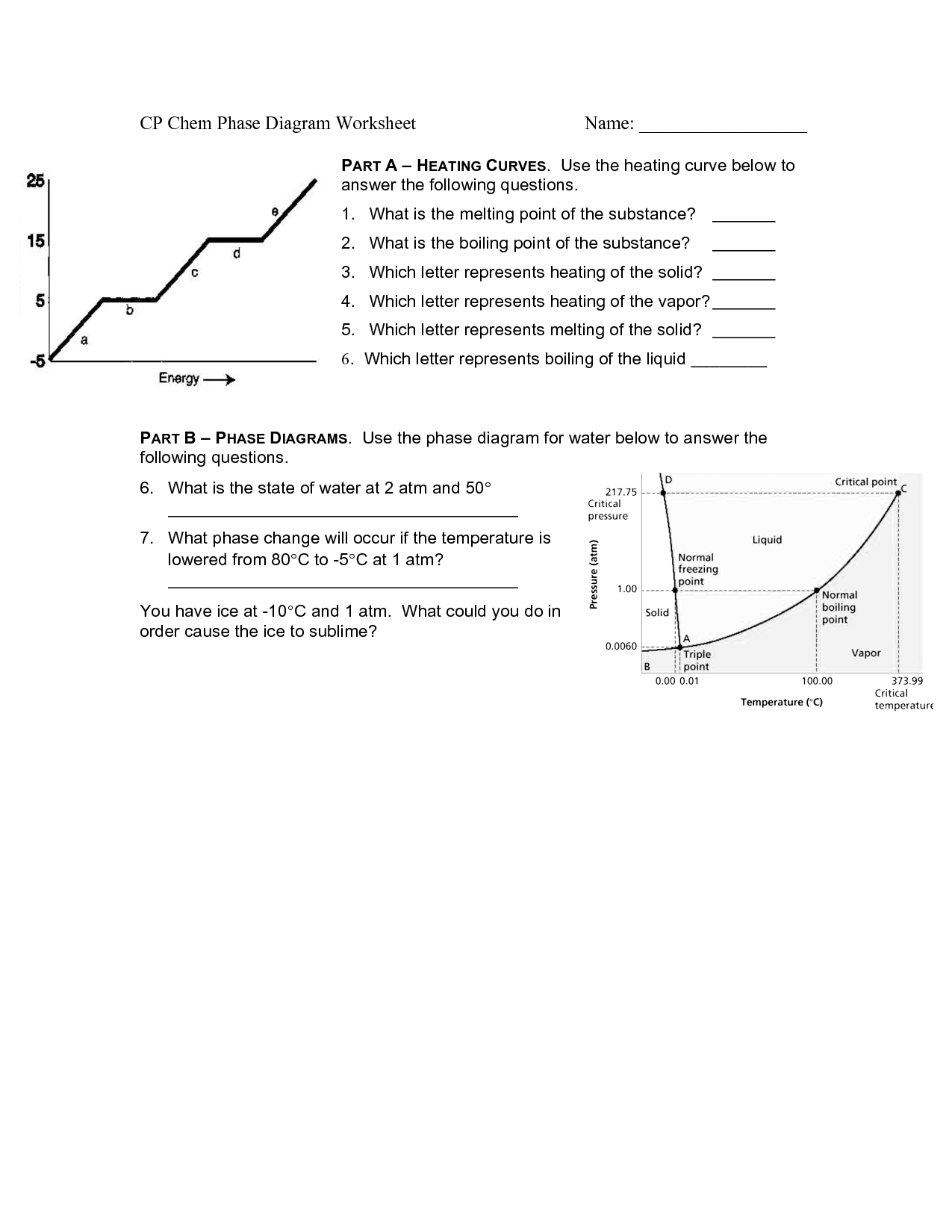
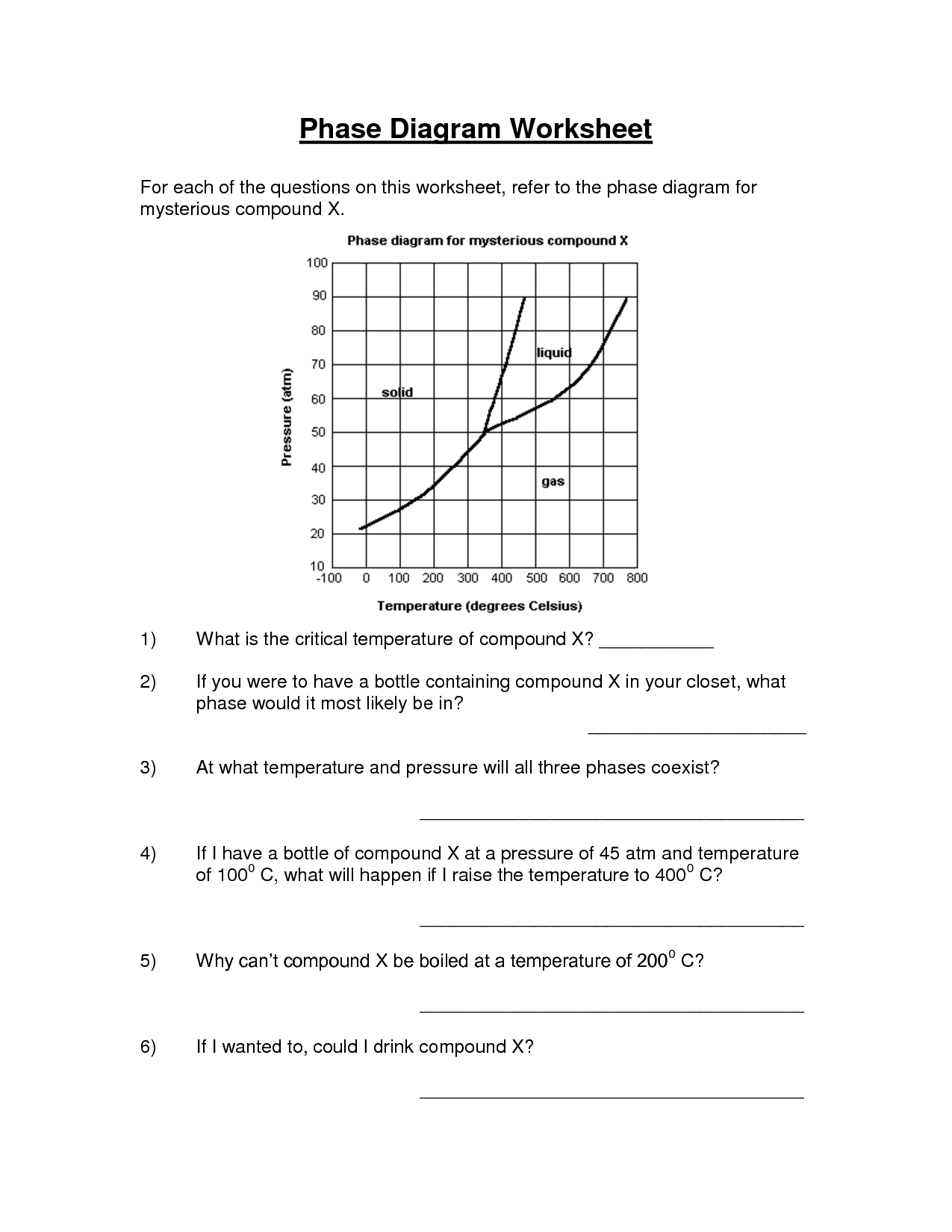
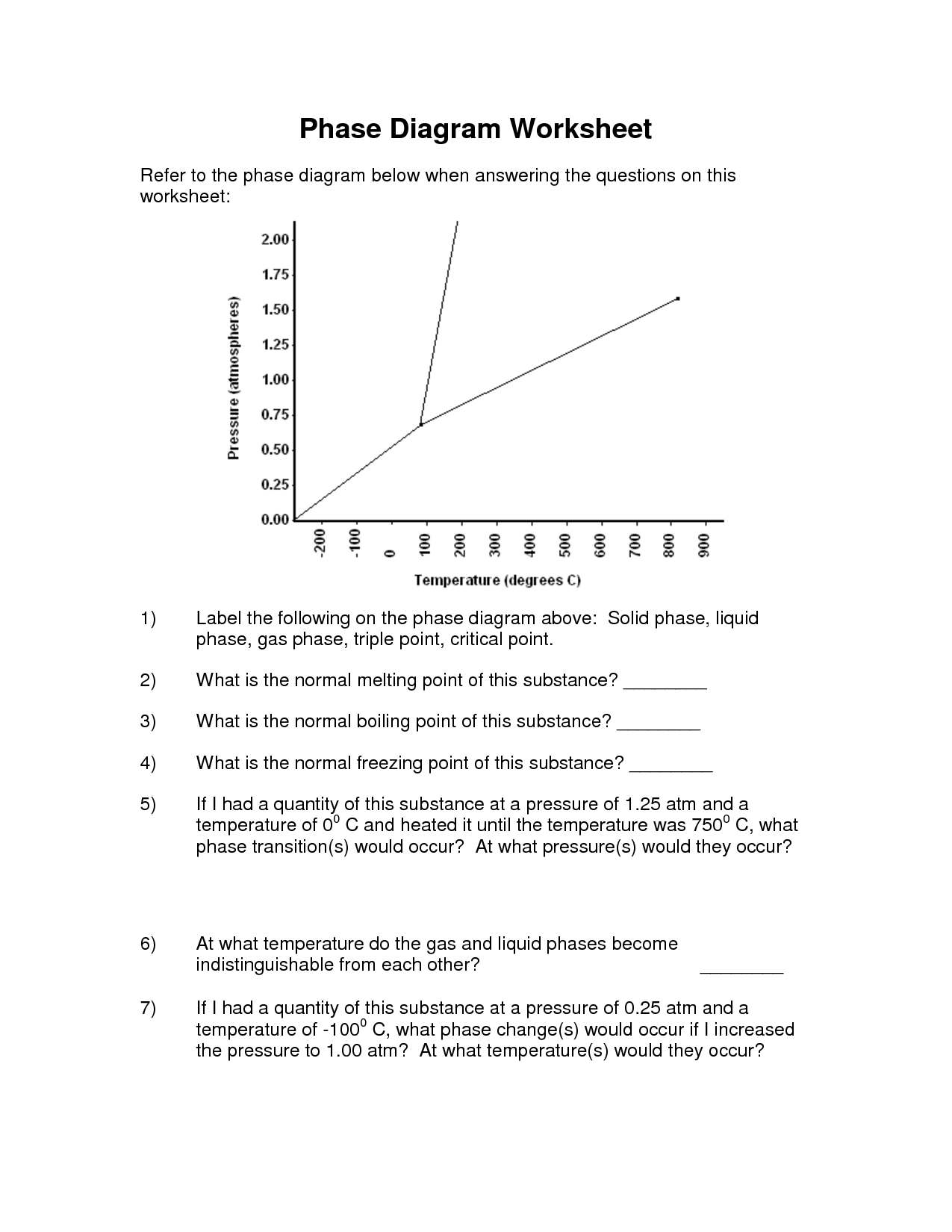
A phase change is a physical change in which a substance transitions from one state of matter (such as solid, liquid, or gas) to another. This transition typically occurs due to changes in temperature or pressure, and involves the absorption or release of energy. Examples of phase changes include melting, freezing, evaporation, condensation, and sublimation.
How does a substance change from one phase to another?
A substance changes from one phase to another through the process of either gaining or losing energy, known as phase transitions. For example, when a solid is heated, it gains energy and its particles begin to vibrate more rapidly, eventually reaching a point where they break free from their fixed positions and form a liquid. This process is known as melting. Similarly, when a liquid is cooled and loses energy, its particles slow down and come together to form a solid, which is called freezing. Other common phase transitions include evaporation (liquid to gas) and condensation (gas to liquid), which are driven by changes in temperature and pressure.
What is the difference between a solid and a liquid?
The main difference between a solid and a liquid lies in their respective states of matter: solids have a fixed shape and volume, with particles arranged in a specific geometric pattern and only vibrating in place, while liquids have a variable shape but a fixed volume, with particles that can move around and flow past each other.
What is the difference between a liquid and a gas?
The main difference between a liquid and a gas lies in their molecular arrangement and interaction. In a liquid, the molecules are close together but still have some degree of freedom to move past each other, giving liquids a definite volume but not a definite shape. On the other hand, in a gas, the molecules are further apart and move more freely, filling the entire container they are in and having neither a definite shape nor volume. This difference in molecular arrangement and movement accounts for the distinctions in physical properties between liquids and gases.
What is sublimation?
Sublimation is a chemical process in which a solid substance transforms directly into a gas without passing through an intermediate liquid state. This process occurs when the vapor pressure of the solid exceeds the surrounding pressure and temperature. Sublimation is commonly seen in substances like dry ice (solid carbon dioxide), mothballs (naphthalene), and certain fragrances or essential oils.
What is deposition?
Deposition is a legal process where witnesses provide sworn testimony outside of court that can be used as evidence in a trial. During a deposition, both parties have the opportunity to ask questions of the witness, who is under oath to tell the truth. The testimony given during a deposition is typically recorded by a court reporter and can be used in court as evidence if the case goes to trial.
What is condensation?
Condensation is the process in which a gas or vapor turns into a liquid. This occurs when the temperature of the gas decreases, causing its particles to slow down and come closer together, leading to the formation of liquid droplets.
What is melting?
Melting is the process in which a solid substance changes into a liquid state due to an increase in temperature. As heat is applied to the solid, its particles absorb energy and start to vibrate more rapidly, causing the substance to break free from its rigid structure and turn into a liquid with less defined shape and volume.
What is freezing?
Freezing is the process in which a liquid changes into a solid state when its temperature is lowered to or below its freezing point. This causes the molecules within the substance to slow down and arrange themselves into a more rigid and ordered structure, resulting in the formation of a solid.
What is vaporization?
Vaporization is the process of a substance changing from a liquid or solid state to a gas or vapor state, typically as a result of being heated to its boiling point. During vaporization, the individual particles of the substance gain enough energy to overcome the forces holding them in a more condensed state, allowing them to break free and spread out as a gas.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.

























Comments