Phase Change Diagram Worksheet Answers
Understanding phase changes and the diagram representing them is essential for students studying chemistry or physics. This worksheet provides the answers to the various questions posed in a phase change diagram activity. It allows students to grasp the concept of how different substances transition between solid, liquid, and gaseous states as temperature and pressure change.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
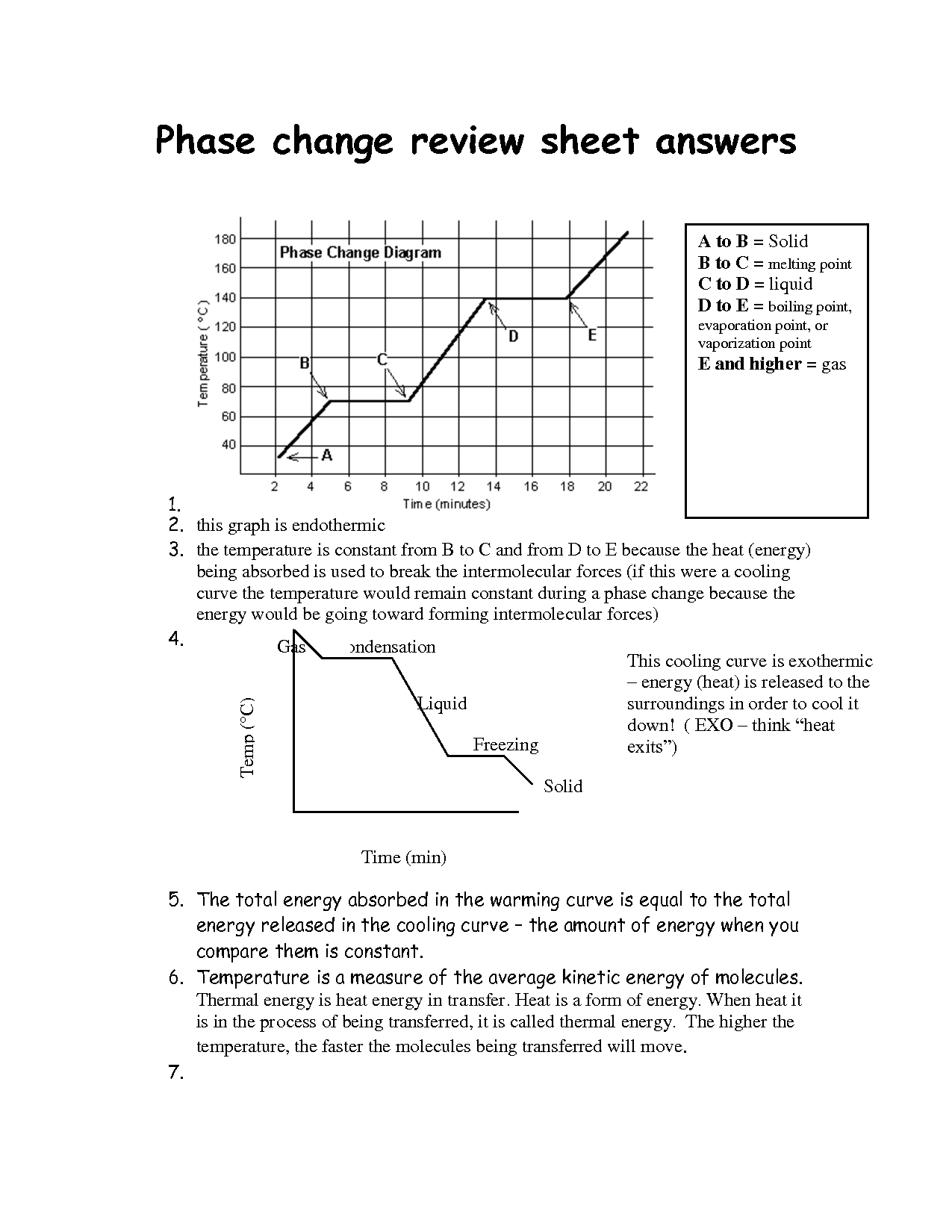
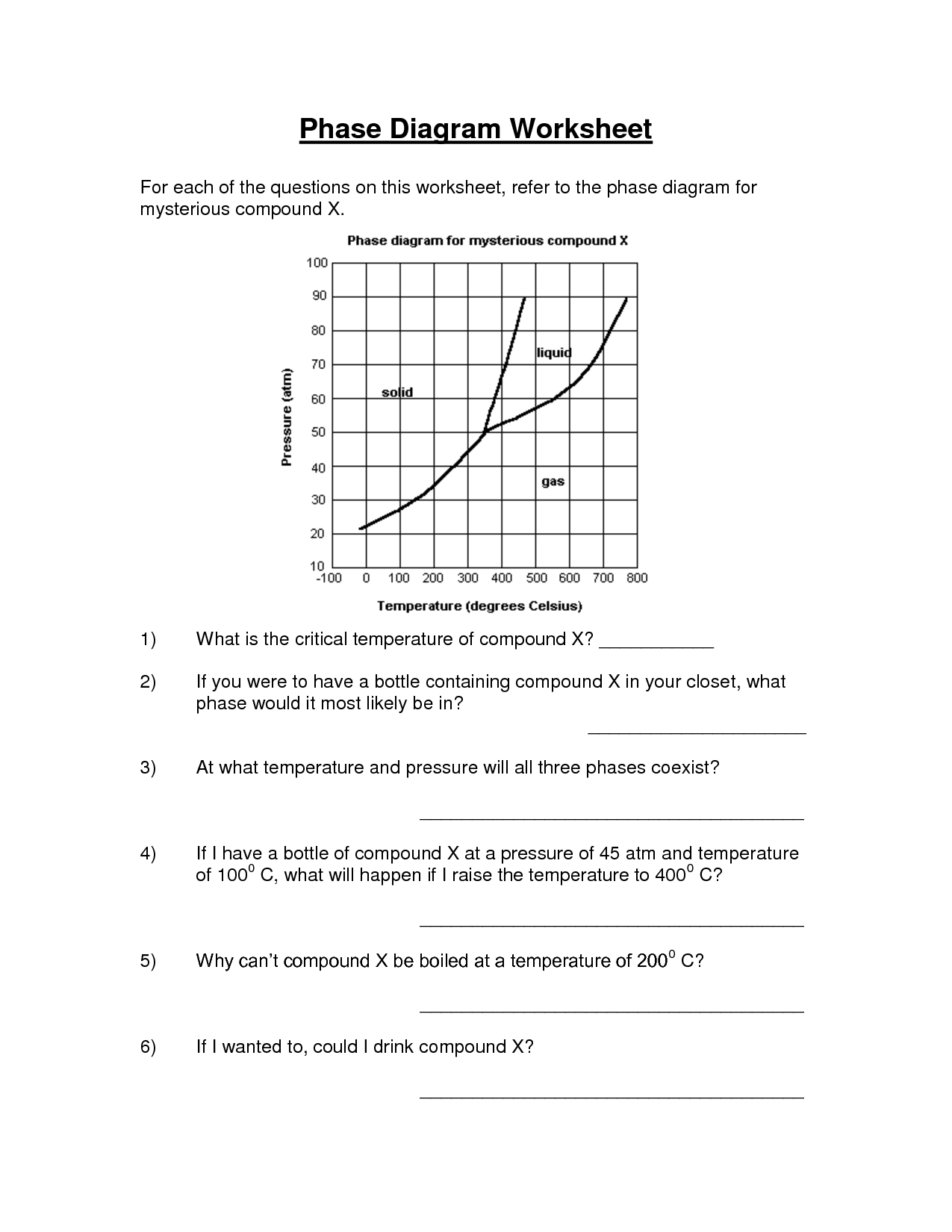
What is a phase change diagram?
A phase change diagram is a graphical representation that shows the physical states of a substance (solid, liquid, gas) at different temperatures and pressures. It illustrates the conditions under which a substance transitions between these states, such as melting, freezing, vaporization, and condensation. The diagram typically includes lines representing the boundaries between different phases and the points at which phase transitions occur, known as critical points.
What are the different phases of matter?
The different phases of matter are solid, liquid, and gas. In a solid, particles are closely packed and have a fixed shape and volume. In a liquid, particles are still close together but can move around, taking the shape of their container. In a gas, particles are far apart and move freely, filling the entire volume of their container. Additionally, there are two other less common phases known as plasma and Bose-Einstein condensate.
Describe the process of melting.
Melting is a phase transition process in which a solid substance changes into a liquid state due to an increase in temperature. When heat energy is applied to a solid material, the molecules gain energy and start vibrating more rapidly, causing the solid structure to break down. As the temperature continues to rise, the bonds holding the molecules in a solid state weaken, allowing the substance to transition into a liquid state where the molecules are free to move past each other. This results in the substance losing its solid shape and becoming a fluid.
Explain the concept of freezing.
Freezing is the process wherein a substance transitions from a liquid state to a solid state due to a reduction in temperature below its freezing point. During freezing, the molecules within the substance slow down and eventually lock into a fixed position, forming a solid structure. This process is characterized by a release of thermal energy as the substance loses heat to its surroundings.
What happens during evaporation?
During evaporation, a liquid substance turns into a gas or vapor state due to an increase in temperature, causing the molecules to gain enough kinetic energy to escape from the surface of the liquid. This process involves the breaking of intermolecular forces holding the liquid together, resulting in molecules transitioning into the gas phase. Evaporation is a cooling process as it removes heat energy from the surroundings, which is why sweating helps in cooling our bodies.
How does condensation occur?
Condensation occurs when warm air, which holds more moisture, comes into contact with a colder surface, causing the air to cool down. As the air temperature drops, it reaches its dew point, the temperature at which the air can no longer hold all the moisture it contains, leading to the formation of tiny water droplets on the colder surface. This process is commonly seen on windows, where the warm indoor air meets the cold glass surface, resulting in the formation of water droplets or frost.
Describe the process of sublimation.
Sublimation is a process where a solid substance changes directly into a gas without passing through the liquid phase. This occurs when the substance is heated to its boiling point and the vapor pressure of the solid exceeds the atmospheric pressure, allowing particles to escape in a vapor form. Sublimation is a physical change, and examples include dry ice turning into carbon dioxide gas and snowflakes evaporating in cold, dry weather conditions.
Explain the term deposition.
Deposition refers to the process where sediments, soil, or rocks are added to a landform or land area. This occurs through erosion, transportation, and settling of particles, leading to the build-up of material. Deposition plays a crucial role in shaping landscapes, such as creating river deltas, forming beaches, and contributing to the formation of sedimentary rock layers over time.
What causes melting point and boiling point to change?
The melting point and boiling point of a substance can change due to factors such as pressure, the presence of impurities, and intermolecular forces. Higher pressure can increase both the melting and boiling point, while impurities can lower these points as they disrupt the orderly arrangement of molecules. Intermolecular forces, such as hydrogen bonding or van der Waals forces, play a significant role in determining these points as stronger forces require higher temperatures to overcome. Additionally, the molecular structure and size of a compound can also affect its melting and boiling points.
How does a phase change diagram help in understanding the behavior of different substances?
A phase change diagram helps in understanding the behavior of different substances by showing the relationship between temperature and pressure at which a substance exists in different phases (solid, liquid, gas). By examining the phase change diagram, one can determine the conditions under which a substance transitions between phases, such as melting, freezing, boiling, or condensing. This information is crucial for predicting and explaining the physical properties and behavior of different substances under varying conditions.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.





















Comments