Molarity Calculations Worksheet
Are you a chemistry student struggling to master molarity calculations? Look no further! This blog post will provide you with a helpful Molarity Calculations Worksheet that will guide you step-by-step in solving problems related to this important topic.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
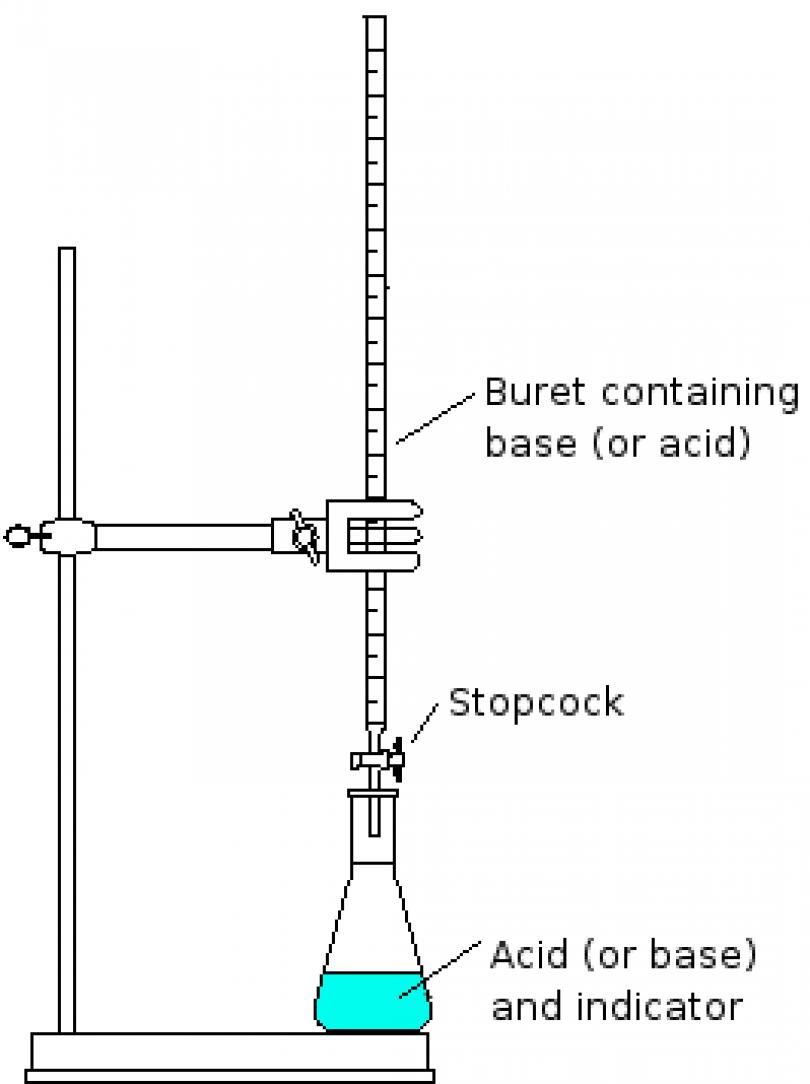
What is molarity?
Molarity is a measure of the concentration of a solute in a solution, expressed as the number of moles of solute per liter of solution. It is widely used in chemistry to quantify the amount of solute in a given volume of solvent, providing a standardized way to compare the concentrations of different solutions.
How is molarity calculated?
Molarity is calculated by dividing the number of moles of solute by the volume of solution in liters. The formula for molarity (M) is: Molarity = moles of solute / liters of solution. This calculation allows for the concentration of a solute in a solution to be expressed in moles per liter, providing a measure of the strength of the solution.
What units are used to express molarity?
Molarity is expressed in units of moles per liter (mol/L) or moles per cubic decimeter (mol/dm^3).
How is the molar mass of a substance determined?
The molar mass of a substance is determined by calculating the sum of the atomic masses of all the atoms in a molecule. This can be done by using the periodic table to find the atomic masses of the elements present in the molecule and then adding them together based on the ratio of each element in the compound. The units for molar mass are grams per mole, and it is a crucial concept in chemistry for converting between mass and moles of a substance.
How does the volume of the solvent affect the molarity of a solution?
The volume of the solvent does not affect the molarity of a solution because molarity is a measure of the concentration of the solute in a given volume of solution. The molarity is solely determined by the amount of solute dissolved in the solution, not by the volume of the solvent. Changing the volume of the solvent will only affect the total volume of the solution, not the molarity of the solute.
How can we convert moles to volume when calculating molarity?
To convert moles to volume when calculating molarity, you can use the formula Molarity (M) = moles of solute (n) / volume of solution in liters (V). Given the moles of solute, you can rearrange the formula to solve for volume: Volume (V) = moles of solute (n) / molarity (M). By dividing the moles of solute by the molarity of the solution, you can determine the volume of the solution in liters.
How can we convert volume to moles when calculating molarity?
To convert volume to moles when calculating molarity, you need to first determine the volume of the solution in liters. Then, use the formula moles = molarity x volume (in liters). By rearranging the formula to solve for moles, you will be able to convert the volume of the solution into moles, which is essential for calculating molarity.
How can dilution be used to change the molarity of a solution?
Dilution can be used to change the molarity of a solution by adding more solvent to decrease the concentration of solute in the solution. When solvent is added to a solution, the total volume increases while the amount of solute remains constant, leading to a decrease in the molarity of the solution. The formula for dilution is M1V1 = M2V2, where M1 and V1 are the initial molarity and volume, and M2 and V2 are the final molarity and volume after dilution, respectively.
What is the formula for dilution?
The formula for dilution is C1V1 = C2V2, where C1 is the initial concentration of the solution being diluted, V1 is the volume of the initial solution being diluted, C2 is the final concentration of the diluted solution, and V2 is the final volume of the diluted solution.
How can we prepare a solution with a desired molarity using a stock solution?
To prepare a solution with a desired molarity using a stock solution, you can use the formula C1V1 = C2V2, where C1 is the initial concentration of the stock solution, V1 is the volume of the stock solution to be used, C2 is the desired final concentration, and V2 is the final volume of the solution you want to prepare. Rearrange the formula to solve for the volume of the stock solution to be used (V1) by dividing (C2V2) by the initial concentration (C1). Measure out this volume of the stock solution and dilute it with solvent (usually water) to reach the desired final volume.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.





















Comments