Intermolecular Forces Worksheet Answers
Are you a student or educator in need of a resource that provides answers to intermolecular forces worksheet questions? Look no further, as this blog post is here to assist you. In this post, we will explore intermolecular forces and provide detailed answers to common questions found in worksheets on this topic. Whether you are studying chemistry or teaching the subject, this blog post will serve as a valuable tool to enhance your understanding of intermolecular forces.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What are intermolecular forces?
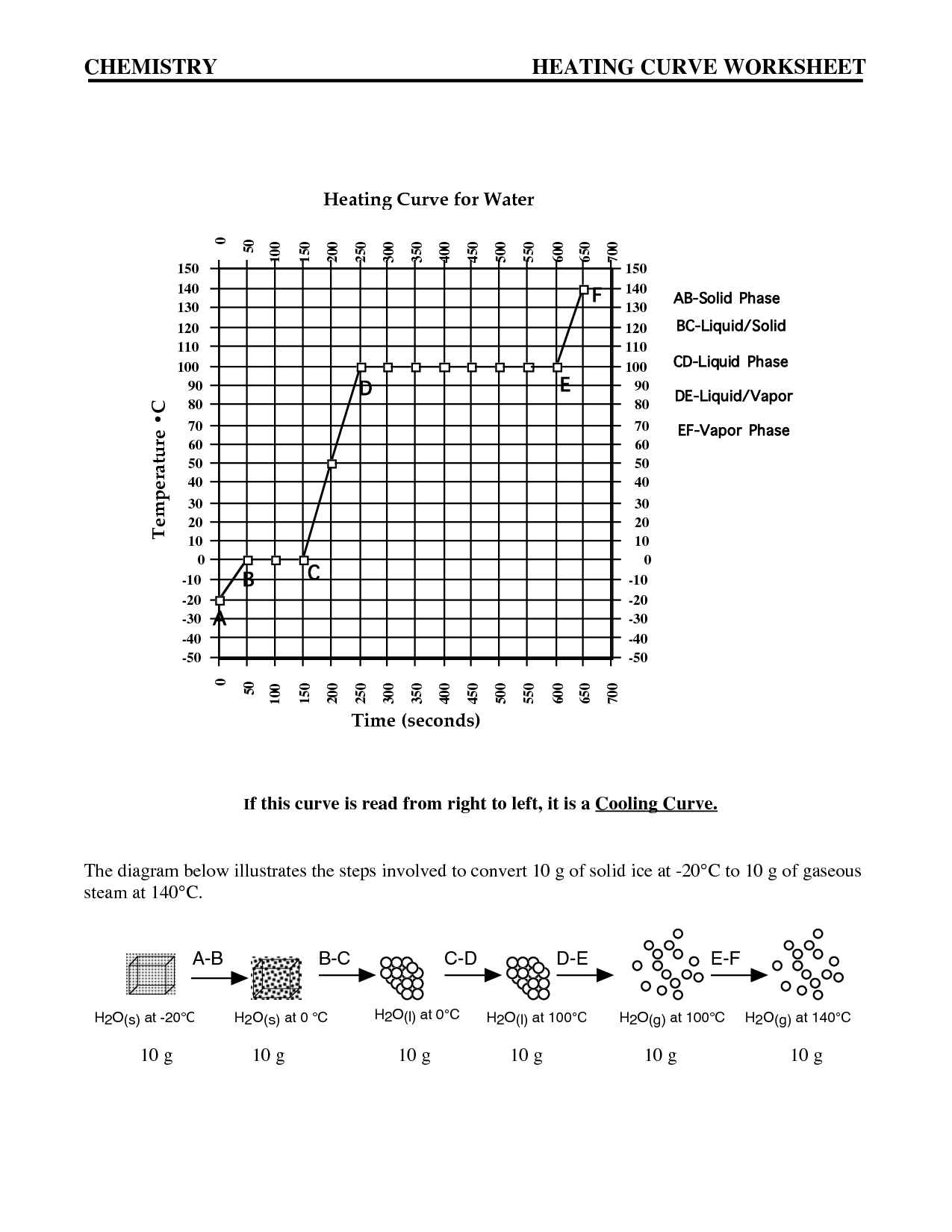
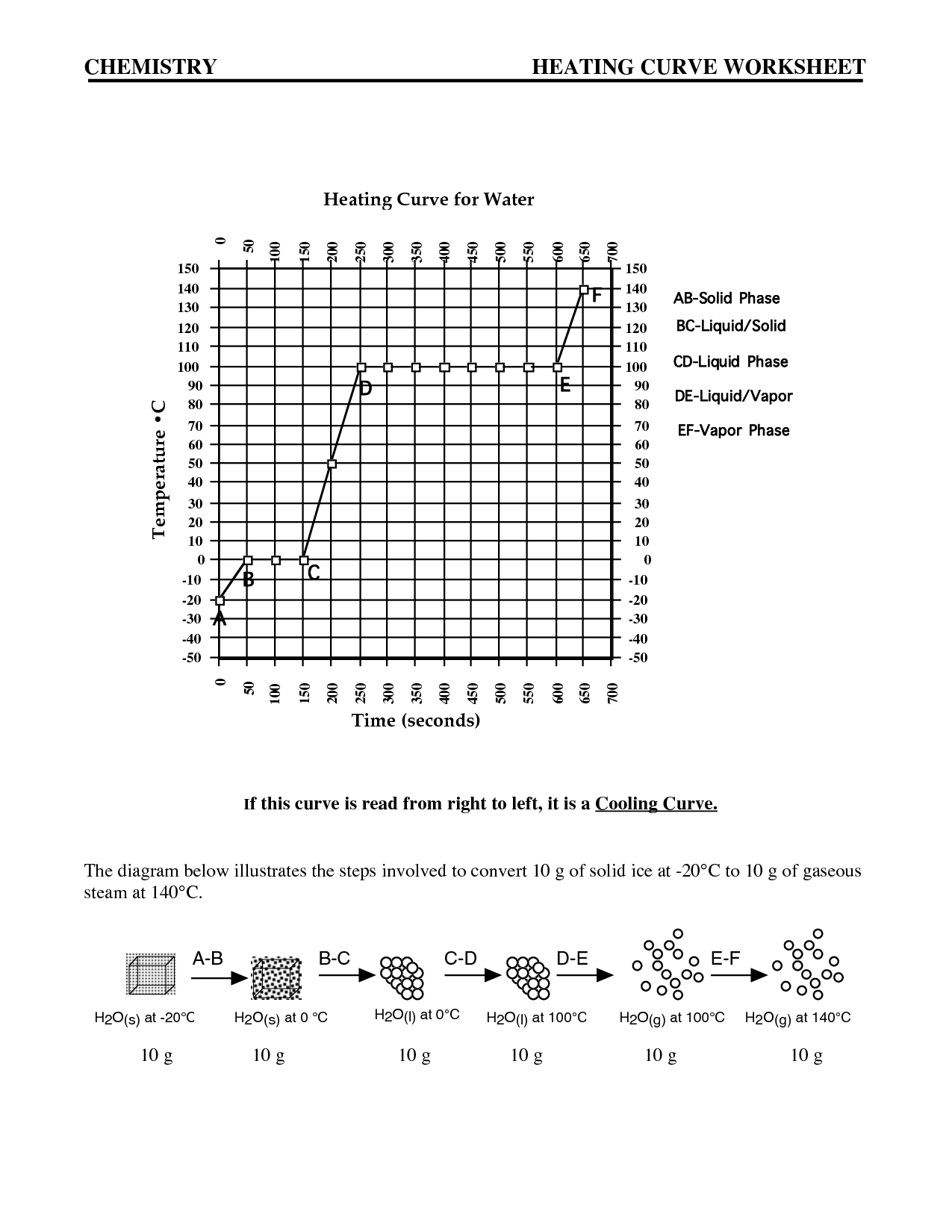
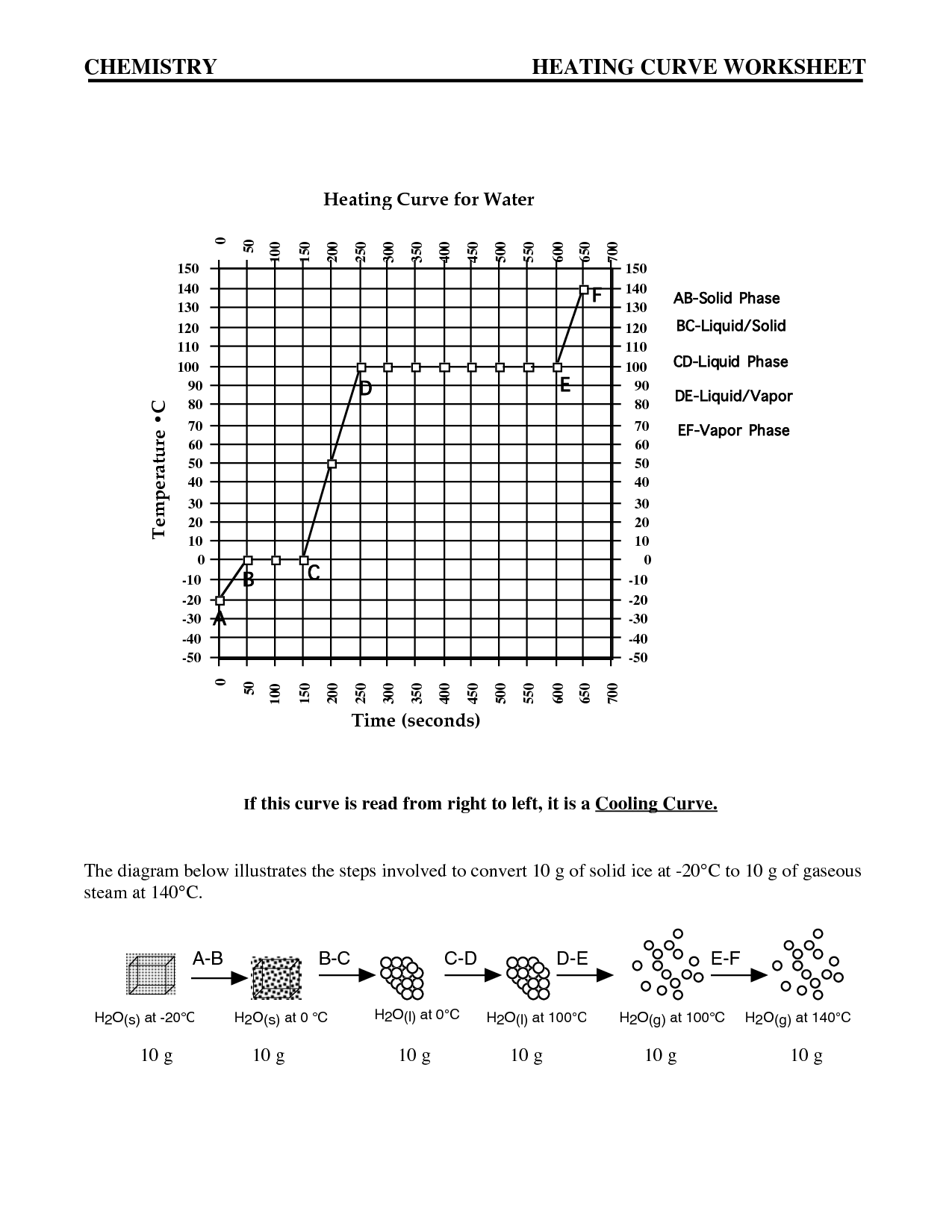
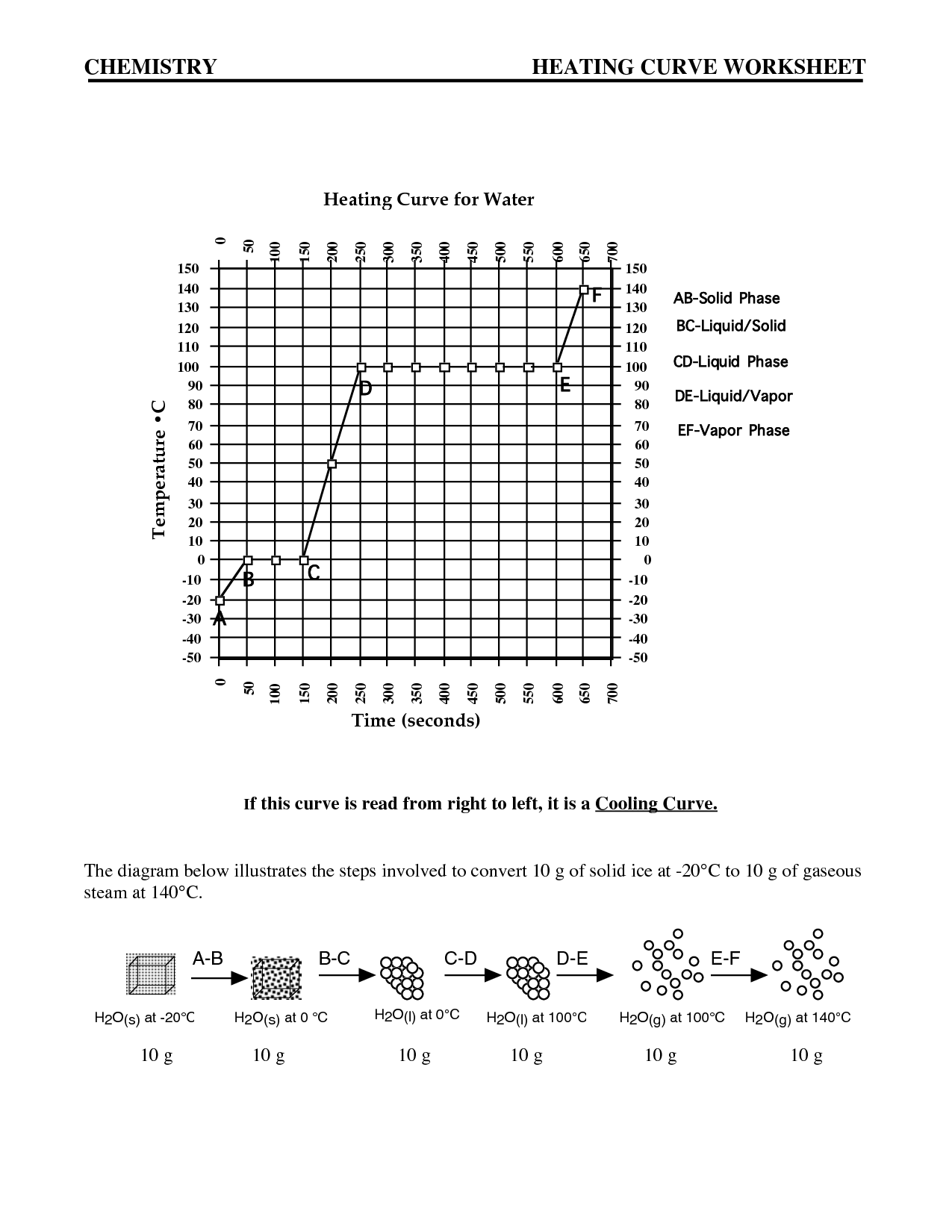
Intermolecular forces are the attractive or repulsive forces that exist between molecules. These forces include hydrogen bonding, dipole-dipole interactions, London dispersion forces, and ion-induced dipole forces. They play a crucial role in determining the physical properties of substances, such as boiling point, melting point, and solubility, by affecting the interactions between molecules in a substance.
What is the difference between intermolecular and intramolecular forces?
Intermolecular forces are the forces of attraction or repulsion between molecules, such as hydrogen bonding, van der Waals forces, and dipole-dipole interactions. In contrast, intramolecular forces refer to the forces that hold atoms together within a molecule, like covalent bonds or ionic bonds. Intermolecular forces determine the physical properties of substances such as boiling point and melting point, while intramolecular forces are responsible for the chemical properties of a compound.
How do London dispersion forces occur?
London dispersion forces occur due to temporary fluctuations in electron distribution within atoms or molecules that lead to the formation of temporary dipoles. These temporary dipoles induce similar fluctuations in neighboring atoms or molecules, creating an attractive force between them. This results in the weak, short-range intermolecular forces known as London dispersion forces.
What is dipole-dipole interaction?
Dipole-dipole interaction is a type of intermolecular force that occurs between molecules with permanent dipoles, meaning they have unequal distribution of charge resulting in a partial positive and partial negative charge. These dipoles attract each other and align in a way that the positive end of one molecule is attracted to the negative end of another, creating a relatively strong force compared to other intermolecular forces such as London dispersion forces. Dipole-dipole interactions contribute to the properties of substances like boiling and melting points, as well as the structure and behavior of many compounds.
How does hydrogen bonding contribute to intermolecular forces?
Hydrogen bonding is a type of intermolecular force that occurs when hydrogen atom is bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) in a molecule, creating a strong dipole-dipole interaction. This results in the attraction between the partially positive hydrogen atom and the partially negative atom of another molecule. Hydrogen bonding is stronger than other types of intermolecular forces like van der Waals forces, and it plays a significant role in determining the physical properties of substances, such as boiling points, melting points, and solubility.
What is the relationship between intermolecular forces and boiling points?
The relationship between intermolecular forces and boiling points is that stronger intermolecular forces generally result in higher boiling points. This is because stronger forces, such as hydrogen bonding or dipole-dipole interactions, require more energy to overcome, leading to a higher temperature needed for the substance to transition from a liquid to a gas phase. Substances with weaker intermolecular forces, like London dispersion forces, typically have lower boiling points as they require less energy to break apart the molecules and transition to a gaseous state.
How do intermolecular forces affect solubility?
Intermolecular forces play a significant role in determining a substance's solubility in a particular solvent. Solubility increases when the intermolecular forces between the solute and solvent are similar in strength, allowing the solute molecules to overcome the attractions holding them together and mix homogeneously with the solvent. Stronger intermolecular forces between solute and solvent typically lead to greater solubility, whereas weak intermolecular forces often result in limited solubility or insolubility. Factors such as hydrogen bonding, dipole-dipole interactions, and London dispersion forces all contribute to how well a substance will dissolve in a particular solvent.
What is the role of intermolecular forces in surface tension?
Intermolecular forces play a crucial role in surface tension by creating a cohesive force among molecules at the surface of a liquid. These forces, such as hydrogen bonding, dipole-dipole interactions, and London dispersion forces, pull the surface molecules inward, creating a resistance to external forces trying to stretch or break the surface. As a result, surface tension is a manifestation of the intermolecular forces holding the liquid molecules together at the surface, allowing the liquid to form droplets and exhibit behaviors like capillary action and the ability to support small objects.
How do intermolecular forces impact viscosity?
Intermolecular forces play a significant role in impacting viscosity by determining how easily molecules can move past each other. Stronger intermolecular forces lead to higher viscosity because molecules are held together more tightly, making it more difficult for them to flow past each other. Conversely, weaker intermolecular forces result in lower viscosity as molecules can move more freely. Overall, the strength of intermolecular forces influences the resistance of a liquid to flow, and therefore affects its viscosity.
How do intermolecular forces affect the physical properties of substances?
Intermolecular forces, such as van der Waals forces, hydrogen bonding, and dipole-dipole interactions, play a significant role in determining the physical properties of substances. These forces affect properties such as melting point, boiling point, viscosity, and solubility. Stronger intermolecular forces result in higher melting and boiling points, as well as higher viscosity and lower solubility. Conversely, weaker intermolecular forces lead to lower melting and boiling points, lower viscosity, and higher solubility. Overall, the type and strength of intermolecular forces greatly influence the behavior and characteristics of a substance.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.





















Comments