Gas Laws Worksheet with Answers
If you're searching for a comprehensive and insightful resource to reinforce your understanding of gas laws, look no further than our Gas Laws Worksheet. Designed to cater to the needs of students and learners of all levels, this worksheet provides a range of questions and exercises that will help you grasp the fundamental concepts pertaining to entities and subjects within the realm of gas laws.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is Boyle's Law?
Boyle's Law states that the pressure of a gas is inversely proportional to its volume when the temperature is constant. This means that as the volume of a gas decreases, its pressure increases, and vice versa. Mathematically, this relationship is expressed as P1V1 = P2V2, where P represents pressure and V represents volume.
What is Charles's Law?
Charles's Law states that for a fixed amount of gas at constant pressure, the volume of the gas is directly proportional to its absolute temperature. In other words, as the temperature of a gas increases, the volume of the gas also increases proportionally, and vice versa. Mathematically, this can be expressed as V1/T1 = V2/T2, where V1 and T1 represent the initial volume and temperature, while V2 and T2 represent the final volume and temperature.
What is Avogadro's Law?
Avogadro's Law states that equal volumes of gases, at the same temperature and pressure, contain the same number of molecules. This means that in a given volume of gas, the number of molecules present is directly proportional to the volume of the gas. This law is a fundamental principle in understanding the behavior of gases and is crucial in the field of chemistry.
What is Gay-Lussac's Law?
Gay-Lussac's Law states that the pressure of a gas is directly proportional to its temperature, provided that the volume and the amount of gas remain constant. This law is commonly expressed as P1/T1 = P2/T2, where P represents pressure and T represents temperature. It demonstrates how changes in temperature can affect the pressure of a gas in a closed system.
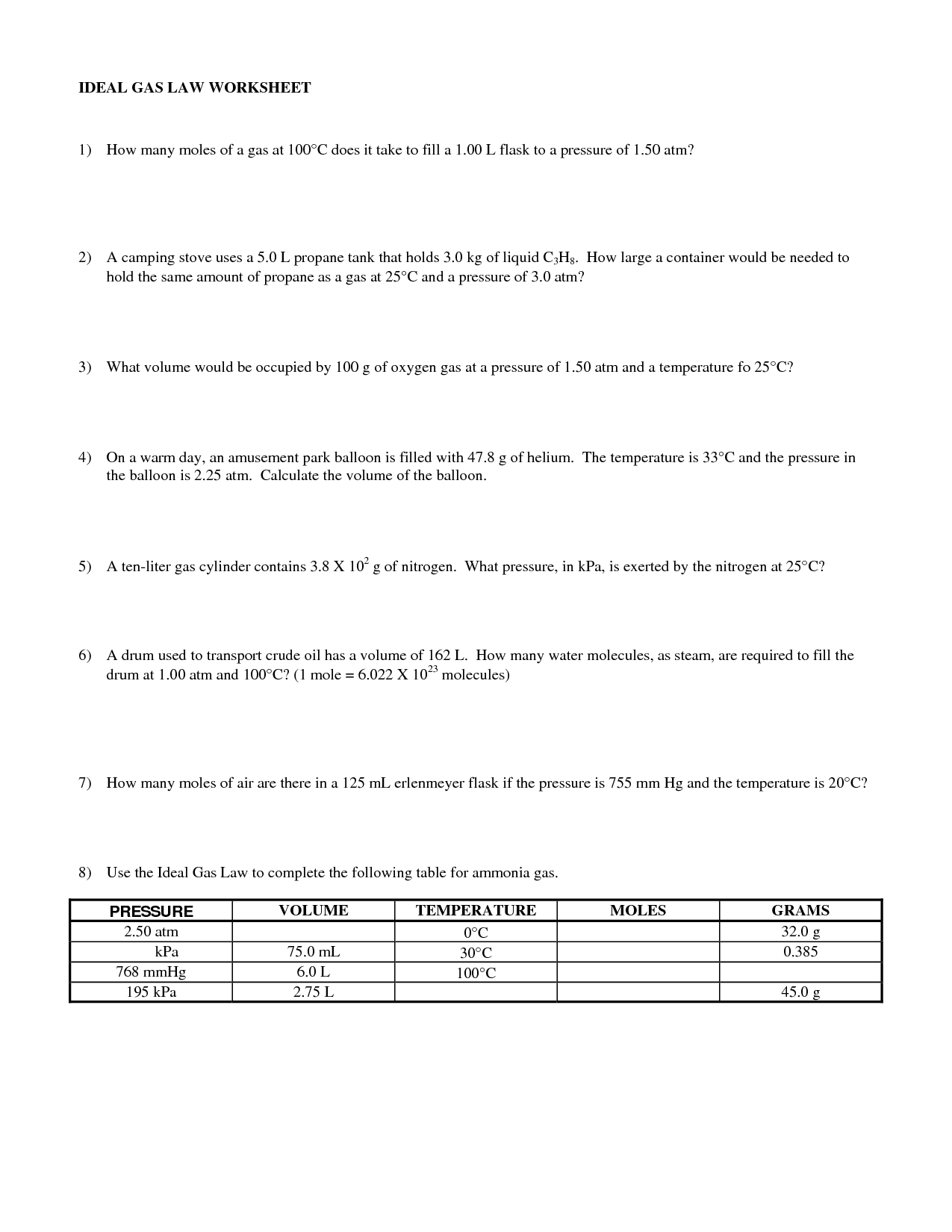
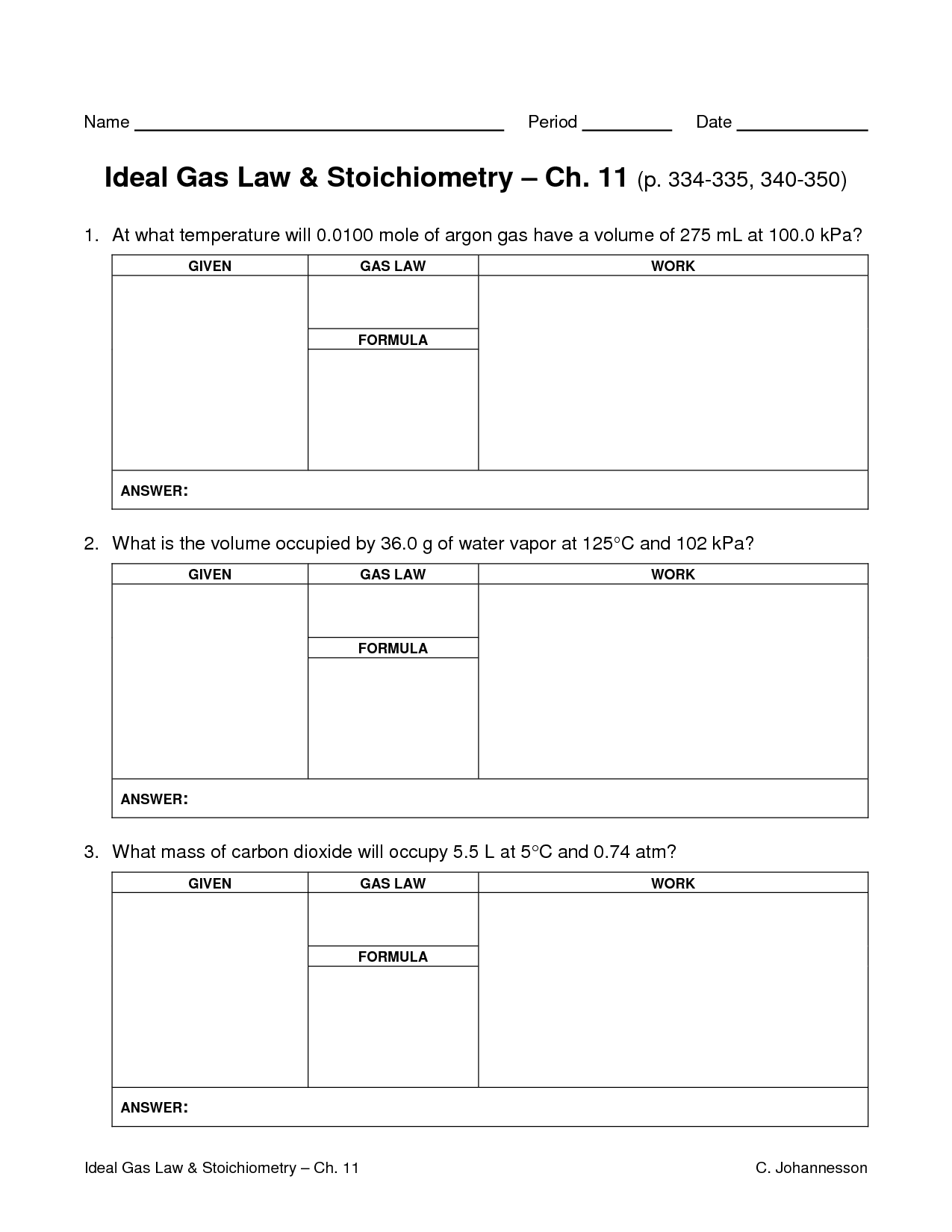
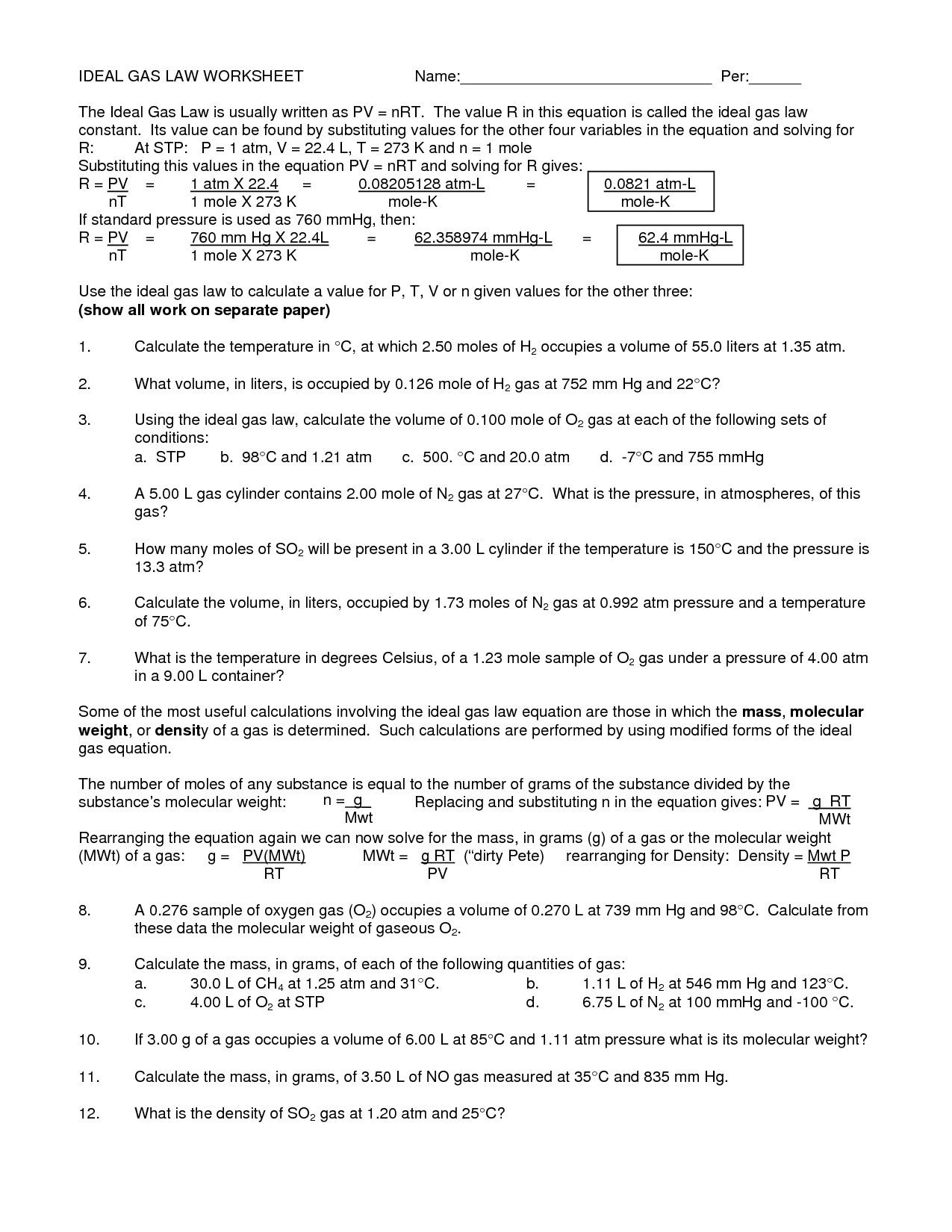
What is the ideal gas law equation?
The ideal gas law equation is PV = nRT, where P represents pressure, V stands for volume, n is the number of moles of the gas, R is the ideal gas constant, and T is the temperature in Kelvin.
What is the relationship between pressure and volume in Boyle's Law?
Boyle's Law states that the pressure of a gas is inversely proportional to its volume when the temperature and amount of gas remain constant. This means that as the volume of a gas decreases, the pressure of the gas increases, and vice versa. Mathematically, this relationship can be expressed as P1V1 = P2V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume, respectively.
What is the relationship between temperature and volume in Charles's Law?
Charles's Law states that the volume of a gas is directly proportional to its temperature, assuming the pressure and amount of gas are constant. This means that as the temperature of a gas increases, its volume will also increase, and vice versa. Charles's Law can be expressed with the formula V1/T1 = V2/T2, where V1 and T1 are the initial volume and temperature, and V2 and T2 are the final volume and temperature.
What is the relationship between volume and moles in Avogadro's Law?
In Avogadro's Law, the relationship between volume and moles of a gas is direct and proportional. This means that as the number of moles of gas increases, the volume of the gas also increases, assuming all other conditions such as temperature and pressure remain constant. This law states that equal volumes of gases, at the same temperature and pressure, contain an equal number of molecules or moles.
What is the relationship between pressure and temperature in Gay-Lussac's Law?
Gay-Lussac's Law states that the pressure of a gas is directly proportional to its temperature, assuming the volume and amount of gas remain constant. This means that as the temperature of a gas increases, so does its pressure, and vice versa. This relationship can be described by the equation P1/T1 = P2/T2, where P represents pressure and T represents temperature.
How can the ideal gas law equation be used to calculate unknown variables in gas problems?
The ideal gas law equation, PV = nRT, can be used to calculate unknown variables in gas problems by rearranging the equation to solve for the specific variable of interest. For example, if you want to find the volume of a gas given the pressure, temperature, and amount of gas, you can rearrange the equation to solve for V (volume) by dividing both sides by the pressure (P) and multiplying by the constant (R) and temperature (T). This allows you to manipulate the equation to find the unknown variable by plugging in the known values of the other variables.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.

































Comments