Empirical Formula Worksheet with Answers
If you're a student or teacher who is in need of practice with empirical formulas, you've come to the right place. This blog post provides an empirical formula worksheet complete with answers to help you sharpen your understanding of this important chemistry concept.
Table of Images 👆
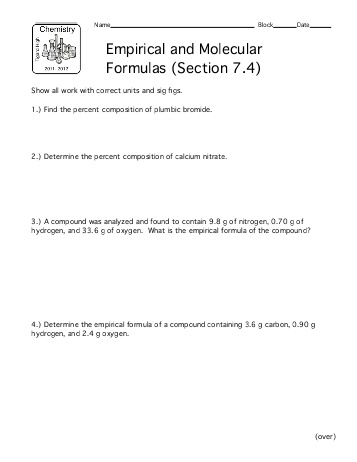
- Molecular and Empirical Formula Worksheet
- Molecular and Empirical Formula Worksheet Answer Key
- Molecular and Empirical Formula Worksheet Answers
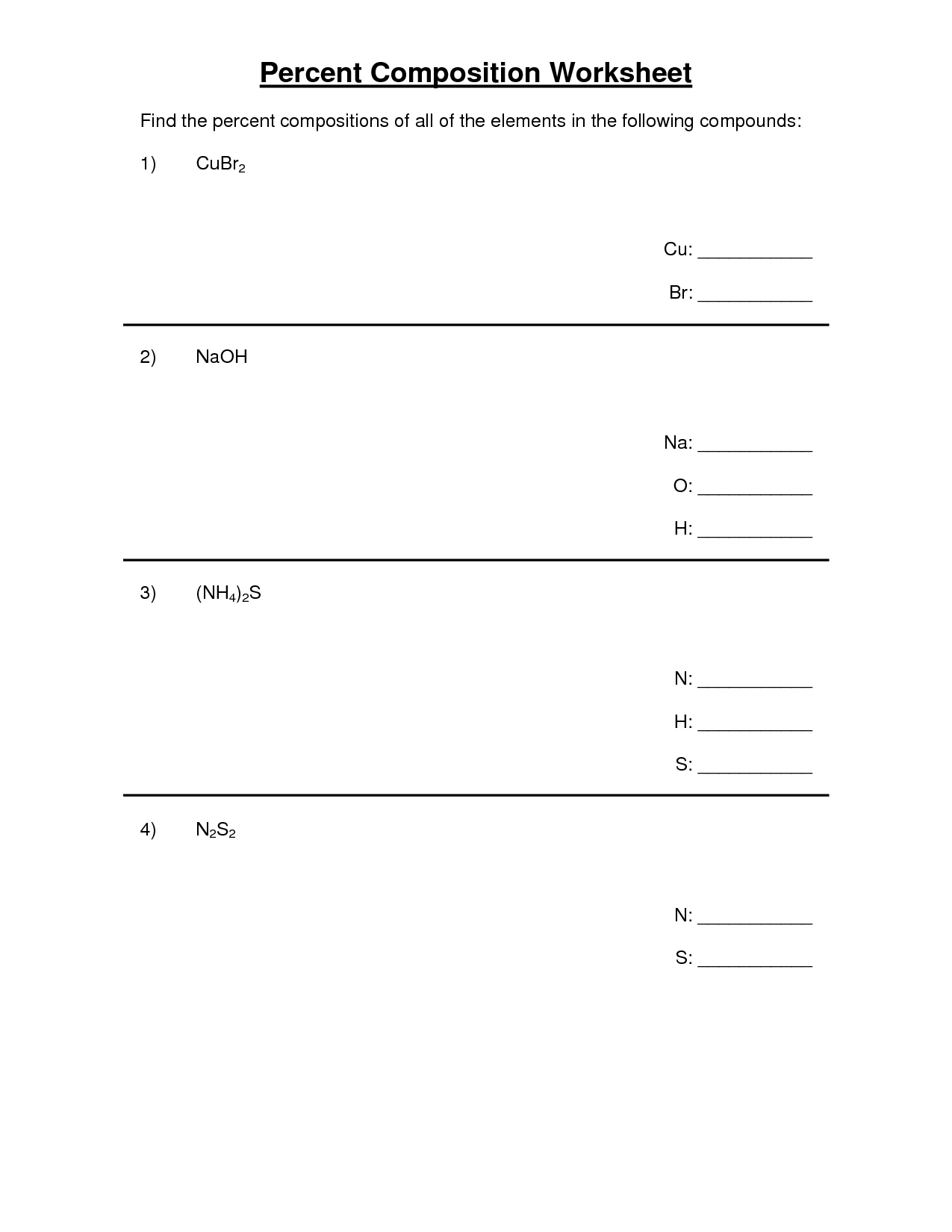
- Percent Composition Worksheet Answer Key
- Percent Composition Empirical Formula Worksheet Answers
- Naming Compounds and Writing Formulas Answers
- Mole Conversion Worksheet
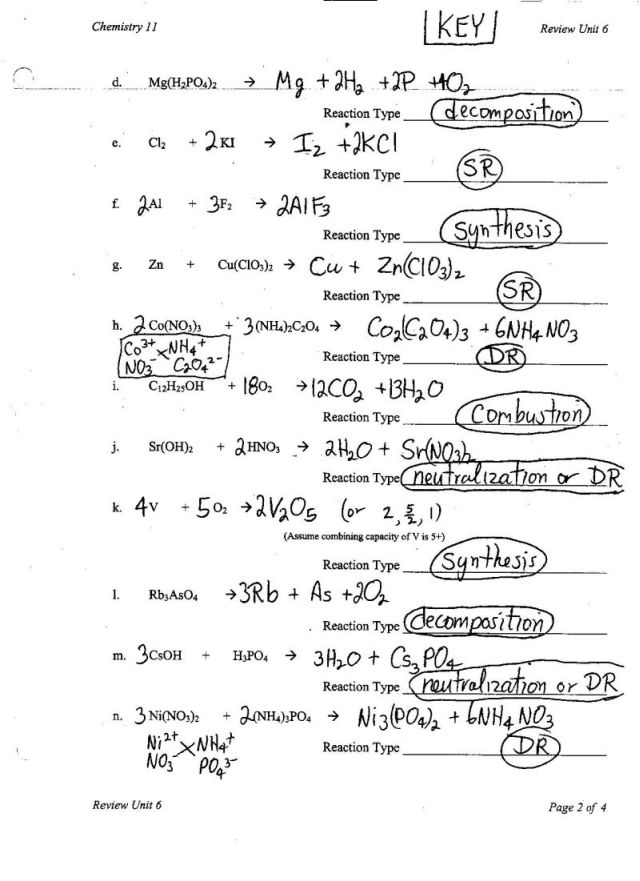
- Types of Chemical Reactions Worksheet Answer Key
- Subtraction to 20 Flash Cards Printable
- Chemical Bonding Worksheet Answer Key
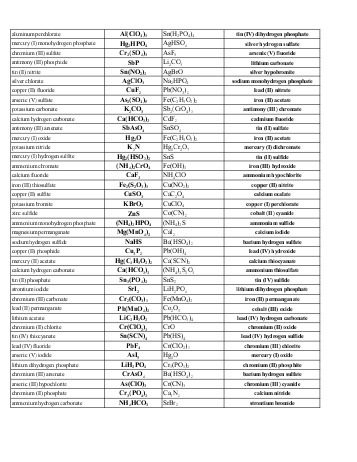
- Naming Ionic Compounds Worksheet Answer Key
- Composition of Blood and Functions Worksheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
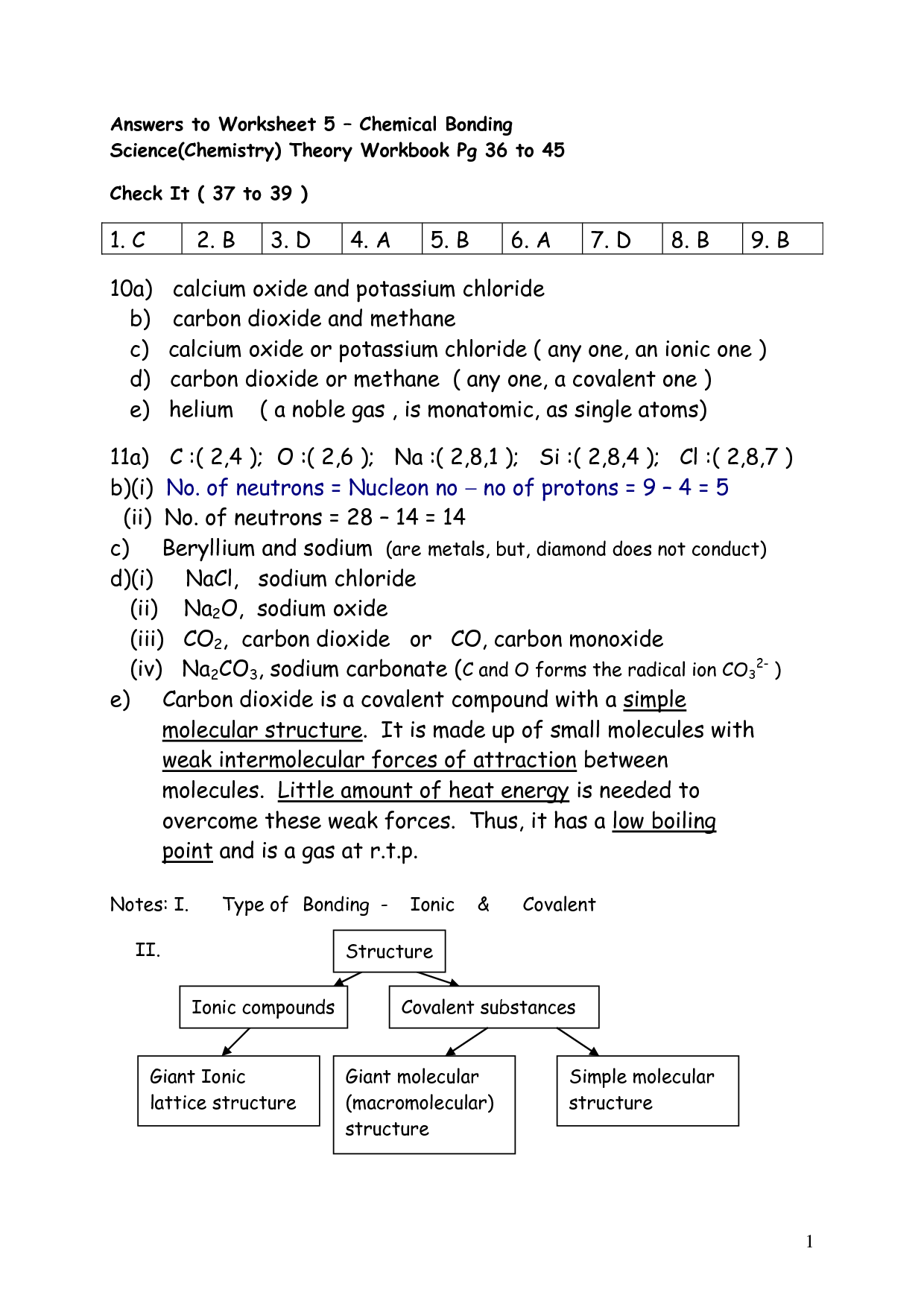
What is the empirical formula of a compound with 65% carbon, 5% hydrogen, and 30% oxygen?
The empirical formula of the compound is CH2O.
C?H?O?
C?H?O? represents the chemical formula for propenoic acid, also known as acrylic acid. It is a clear, colorless liquid with a pungent smell and is commonly used in the production of plastics, adhesives, and coatings.
What is the empirical formula of a compound that contains 40% carbon, 6.7% hydrogen, and 53.3% oxygen?
The empirical formula of the compound would be CH2O, as it represents the simplest whole-number ratio of the elements present in the compound.
C?H?O
C?H?O represents the chemical formula for acetate ion. The acetate ion has a charge of -1 and is commonly found in salts such as sodium acetate and calcium acetate. It is also a key component in acetic acid, which is present in vinegar.
A compound is found to contain 26.7% nitrogen and 73.3% oxygen. What is its empirical formula?
The empirical formula of the compound containing 26.7% nitrogen and 73.3% oxygen is NO2. This can be determined by converting the percentages to moles (assuming a 100g sample) and then finding the simplest whole-number ratio between nitrogen and oxygen atoms, which in this case is 1:2.
If a compound contains 46.7% carbon, 6.7% hydrogen, and 46.6% oxygen, what is its empirical formula?
To find the empirical formula, you need to convert the percentages into moles. Then, divide each mole value by the smallest number of moles calculated to obtain whole number ratios. After converting the moles to whole numbers, the empirical formula of the compound with 46.7% carbon, 6.7% hydrogen, and 46.6% oxygen would be CH2O.
A compound contains 80% carbon and 20% hydrogen. What is its empirical formula?
The empirical formula of the compound is CH4, as it would have one carbon atom and four hydrogen atoms per molecule based on the given percentages of elements.
A compound contains 37.5% carbon, 12.5% hydrogen, and 50% oxygen. What is the empirical formula?
The empirical formula of the compound is CH2O, which can be determined by converting the percentage composition into moles and finding the simplest whole-number ratio of the elements present.
C?H?O
C?H?O represents the chemical formula for Acetaldehyde, a colorless, flammable liquid with a pungent, fruity odor. It is commonly used in the production of plastics, chemicals, and pharmaceuticals.
A compound is composed of 40% carbon and 60% oxygen. What is its empirical formula?
The empirical formula of the compound is CO3.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.
























Comments