Emission Spectra Worksheet
Are you a science teacher searching for an engaging and educational activity for your students? Look no further than our Emission Spectra Worksheet. Designed specifically for high school students, this worksheet provides a comprehensive overview of emission spectra and their significance in the field of physics. The worksheet explores the concept of electron transitions, the energy levels involved, and the resulting wavelengths of light emitted. With clear explanations and thought-provoking questions, this worksheet is the perfect resource to reinforce your students' understanding of this fascinating topic.
Table of Images 👆
- Element Emission Spectra Worksheet
- Emission Line Spectrum Worksheet
- Atomic Model Timeline Worksheet
- Atomic Spectra Worksheet
- Bright Line Spectrum Chemistry
- Bright Line Emission Spectrum
- Bohr Atomic Models Worksheet Answers
- Emission Spectra Lab Answers
- Printable Color Theory Worksheet
- Emission Spectroscopy Chemistry Lab
- Chemistry Worksheet Matter 1
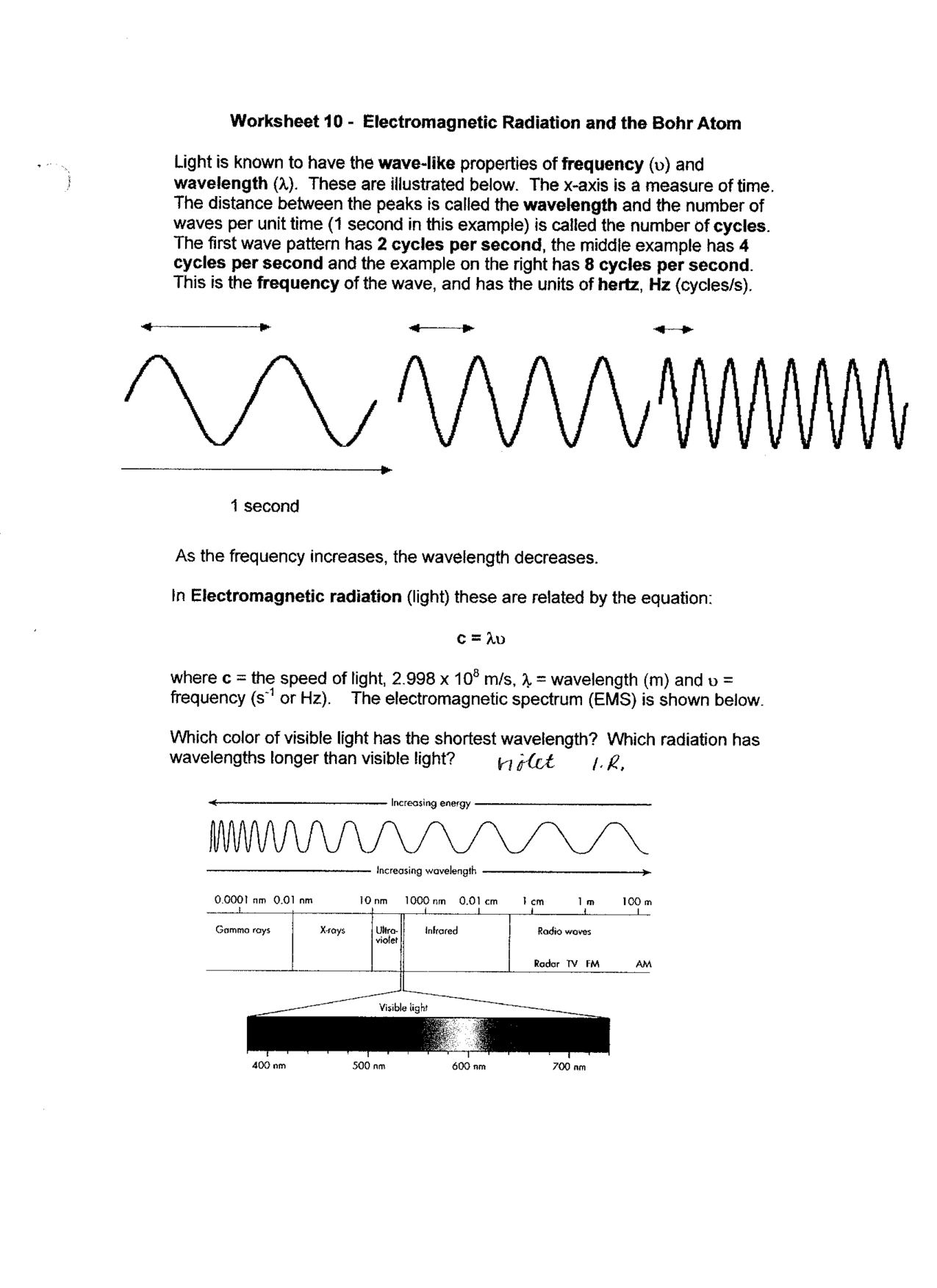
- Waves and Electromagnetic Spectrum Worksheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
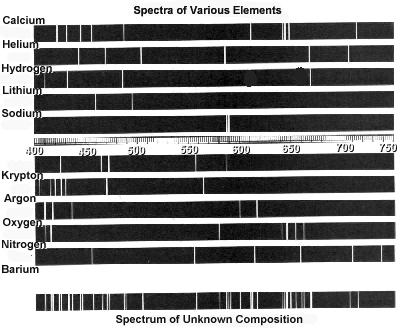
What is an emission spectrum?
An emission spectrum is a collection of spectral lines produced when atoms or molecules emit photons as they transition from excited states to lower energy states. Each element or molecule has a unique emission spectrum, often used for analytical purposes like identifying elements present in a sample or studying the energy levels of atoms or molecules.
What is the relationship between emission spectra and atoms?
Emission spectra are unique patterns of light emitted by atoms or molecules when they transition from higher to lower energy levels. Atoms emit light at specific wavelengths that are characteristic of the particular element, making emission spectra a valuable tool for identifying elements based on the spectral lines they produce. The relationship between emission spectra and atoms lies in the fact that the discrete energy levels of atoms result in specific and identifiable spectral lines, providing insight into the atomic structure and composition of matter.
How is an emission spectrum different from an absorption spectrum?
An emission spectrum is produced when an atom or molecule emits electromagnetic radiation as it transitions from excited states to lower energy states, resulting in bright lines on a dark background, whereas an absorption spectrum is produced when an atom or molecule absorbs specific wavelengths of light, resulting in dark lines on a continuous spectrum. In other words, an emission spectrum shows the specific wavelengths of light emitted by a substance, while an absorption spectrum shows the wavelengths of light that are absorbed by the substance.
What causes the lines or bands in an emission spectrum?
The lines or bands in an emission spectrum are caused by the specific wavelengths of light emitted when electrons in an atom or molecule transition from higher energy levels to lower energy levels. Each element or molecule has its unique set of energy levels, leading to distinct patterns of lines or bands in their emission spectra. The differences in energy levels result in the emission of light at specific wavelengths, creating the characteristic lines or bands observed in the spectrum.
Can each element have its own unique emission spectrum?
Yes, each element can have its own unique emission spectrum because the arrangement of electrons in different elements leads to specific energy transitions when they absorb and release energy. This results in distinct patterns of light emitted by each element, making it possible to identify elements based on their unique emission spectra.
How is the energy of emitted light related to the lines in an emission spectrum?
The energy of emitted light is directly related to the specific lines observed in an emission spectrum. Each line in the emission spectrum corresponds to a specific energy difference between discrete energy levels of the atoms or molecules emitting the light. When an electron transitions from a higher energy level to a lower one, a photon is emitted with an energy equal to the difference in energy between the two levels. Therefore, the distinct lines in an emission spectrum represent the different energy transitions that occur within the atom or molecule, providing information about its electronic structure.
How can emission spectra be used to identify elements?
Emission spectra can be used to identify elements by analyzing the unique set of spectral lines emitted by each element when it is excited. Each element emits a distinct pattern of wavelengths of light when its electrons move from higher energy levels to lower energy levels. By comparing these emission spectra to known patterns or databases, scientists can accurately identify the elements present in a sample based on the wavelengths of light that are emitted.
What is the significance of spectral lines in emission spectra?
Spectral lines in emission spectra are significant because they represent unique wavelengths of light that are emitted when electrons in an atom or molecule move from higher energy levels to lower energy levels. These lines are characteristic of the elements or compounds emitting the light, allowing scientists to identify the composition of a substance. By analyzing the patterns and positions of these spectral lines, researchers can gain valuable insights into the structure, behavior, and properties of matter at the atomic and molecular levels.
How do the positions and intensities of spectral lines vary in an emission spectrum?
In an emission spectrum, the positions of spectral lines are determined by the elements present in the sample, with each element having its unique set of spectral lines. The intensities of these spectral lines vary based on factors such as the concentration of the element in the sample, the temperature of the source, and the energy transitions within the atom. Generally, the intensity of spectral lines in an emission spectrum is proportional to the amount of energy released during the transition of electrons between energy levels in the atom.
Can emission spectra be used to determine the composition of a substance?
Yes, emission spectra can be used to determine the composition of a substance. When a substance is heated or excited, it emits light at specific wavelengths based on its unique atomic or molecular composition. By analyzing the emitted light using spectrometry techniques, scientists can identify the elements or compounds present in the substance. This method is commonly used in fields such as chemistry, physics, and astronomy to determine the composition of unknown substances.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.

























Comments