Basic Atomic Structure Worksheet Key
Are you teaching your students about the basic atomic structure? If so, you may be searching for a helpful worksheet that covers the essential concepts. Look no further! We have a key for a Basic Atomic Structure Worksheet that will guide your students in understanding the entity and subject of atoms in a simple and clear manner.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
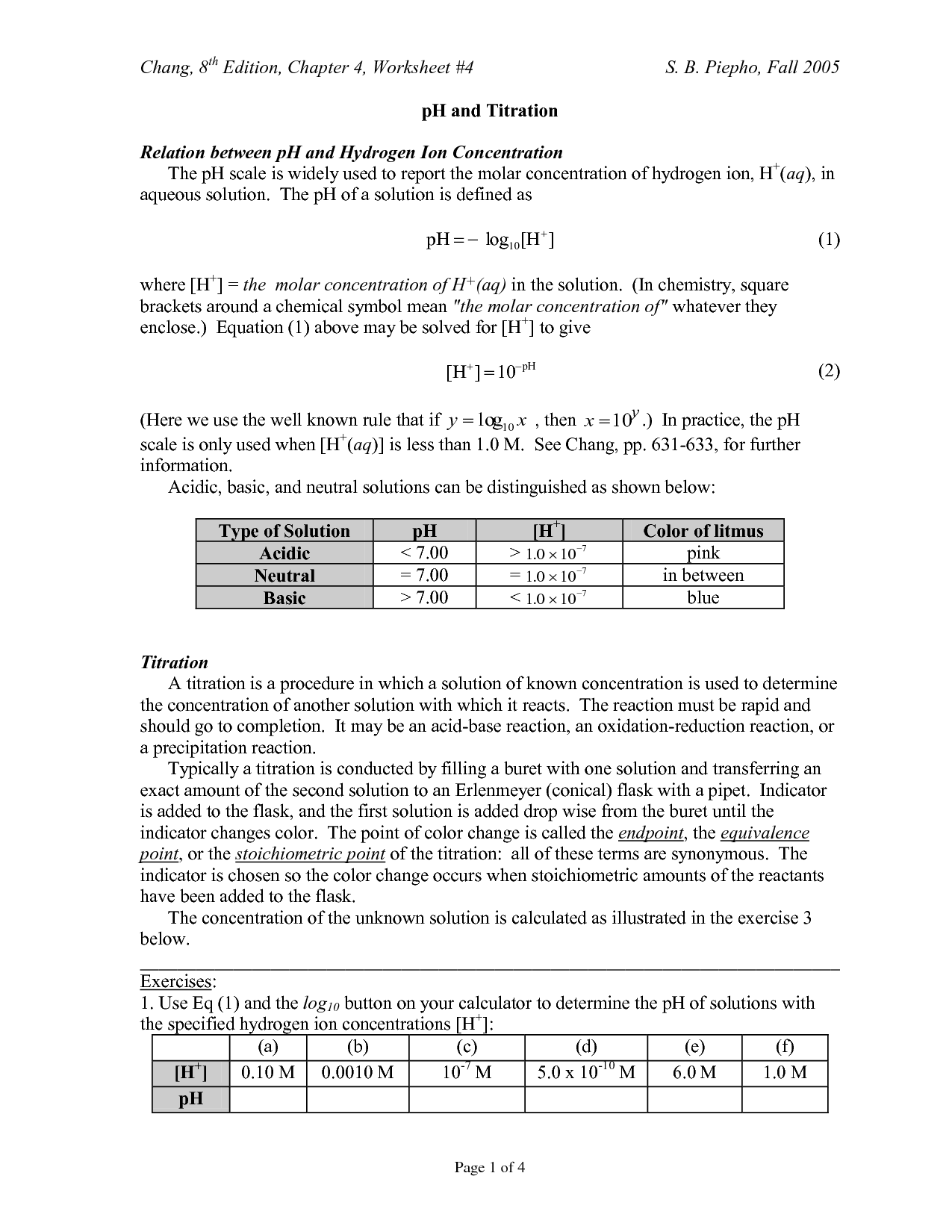
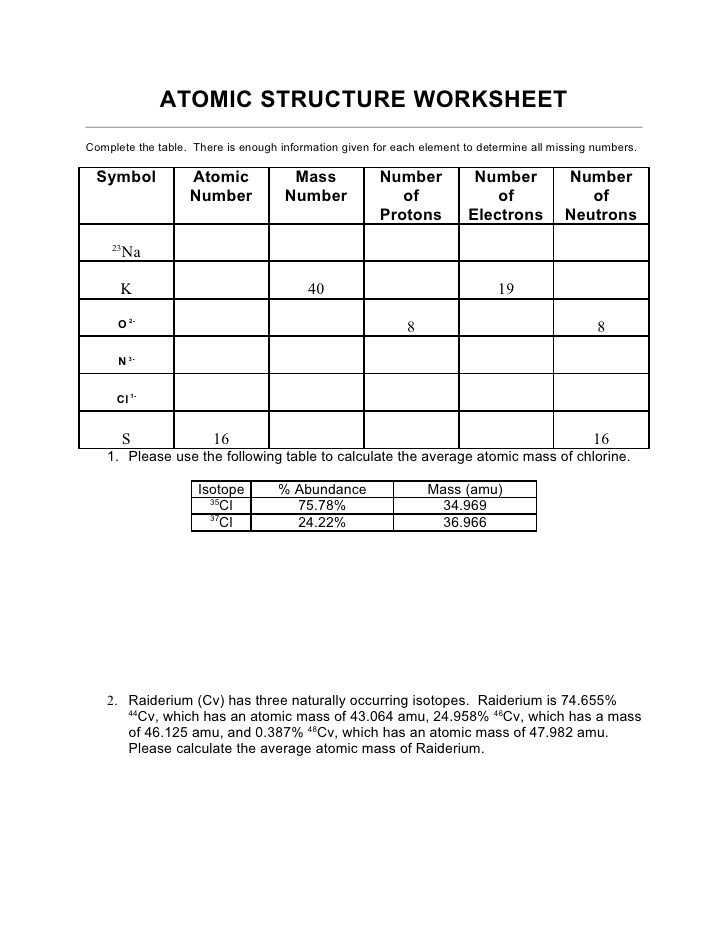
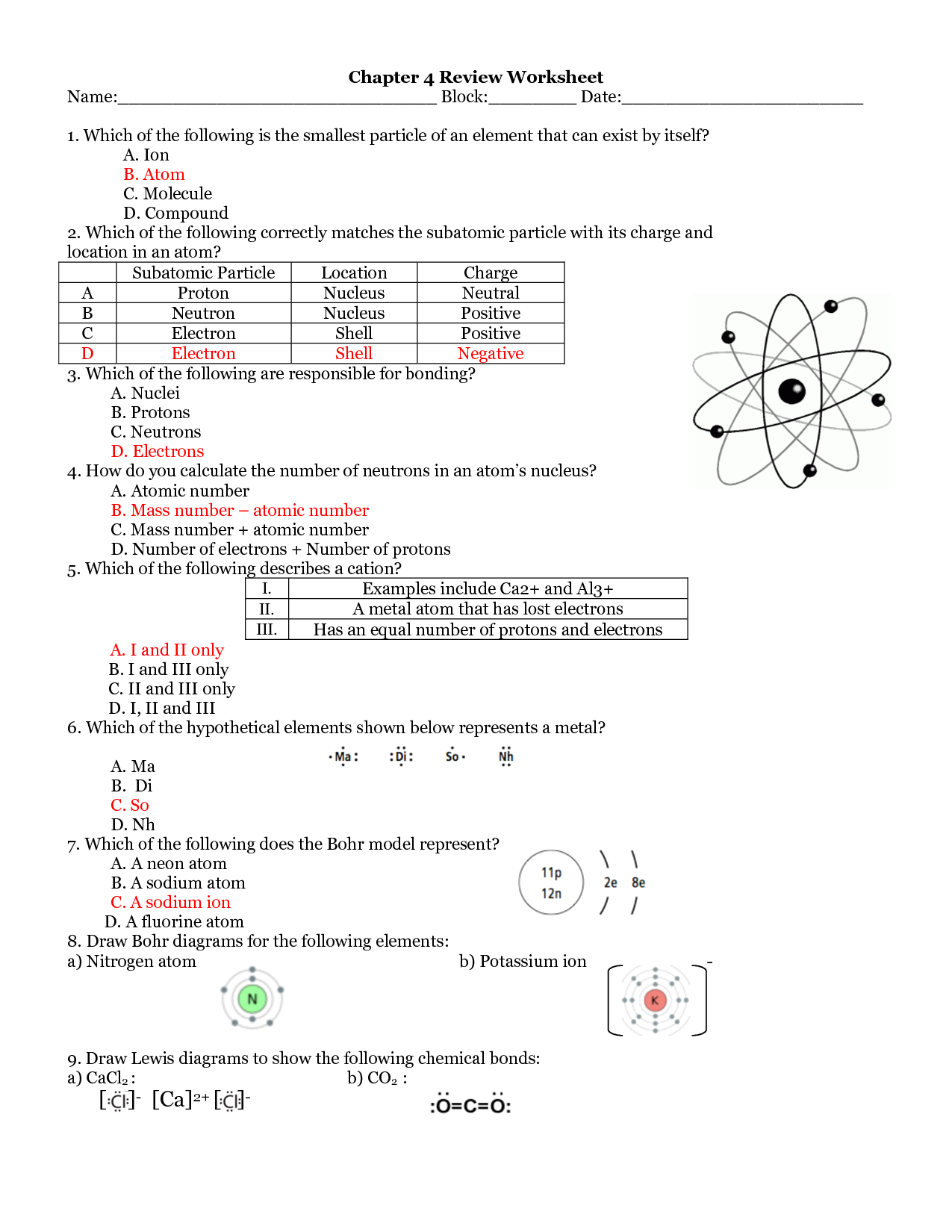
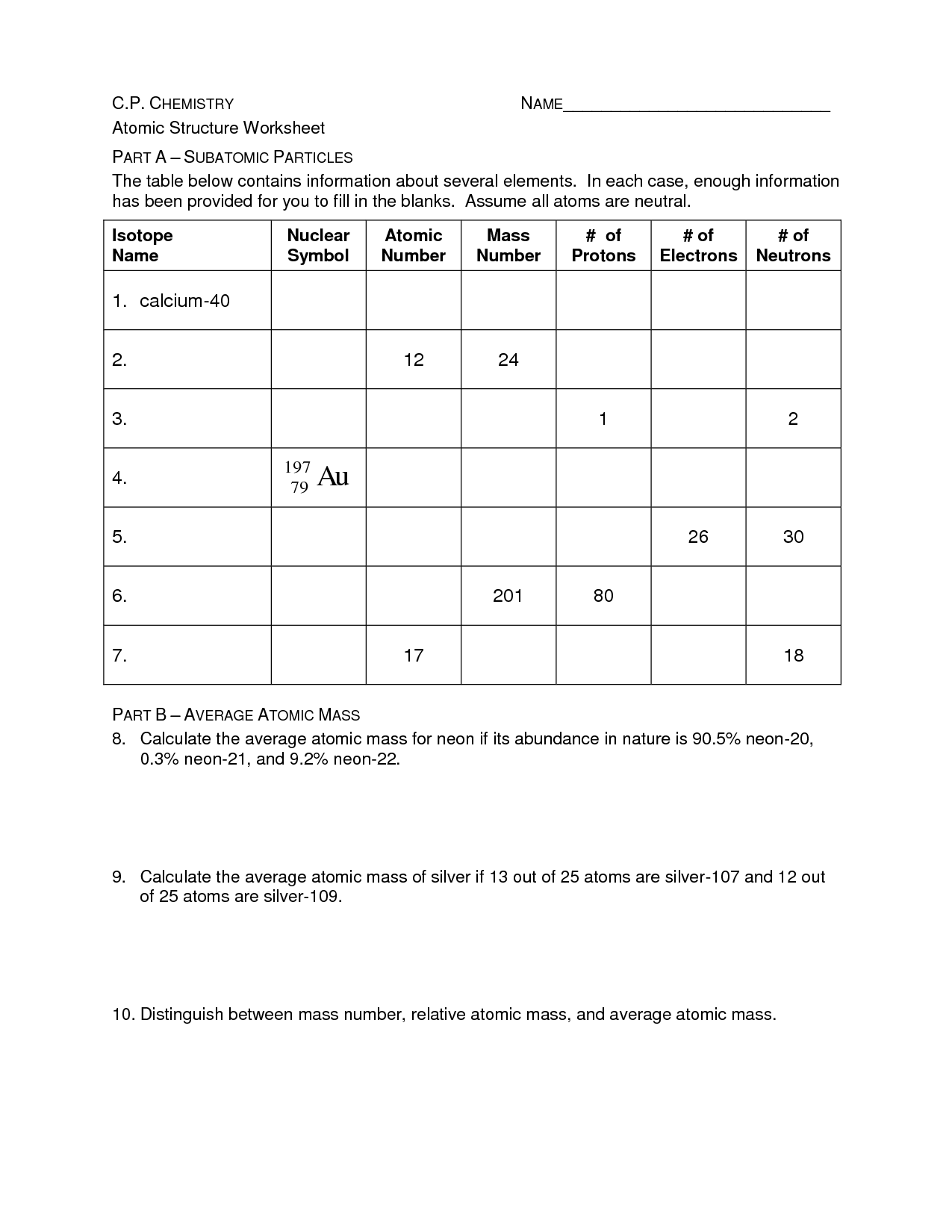
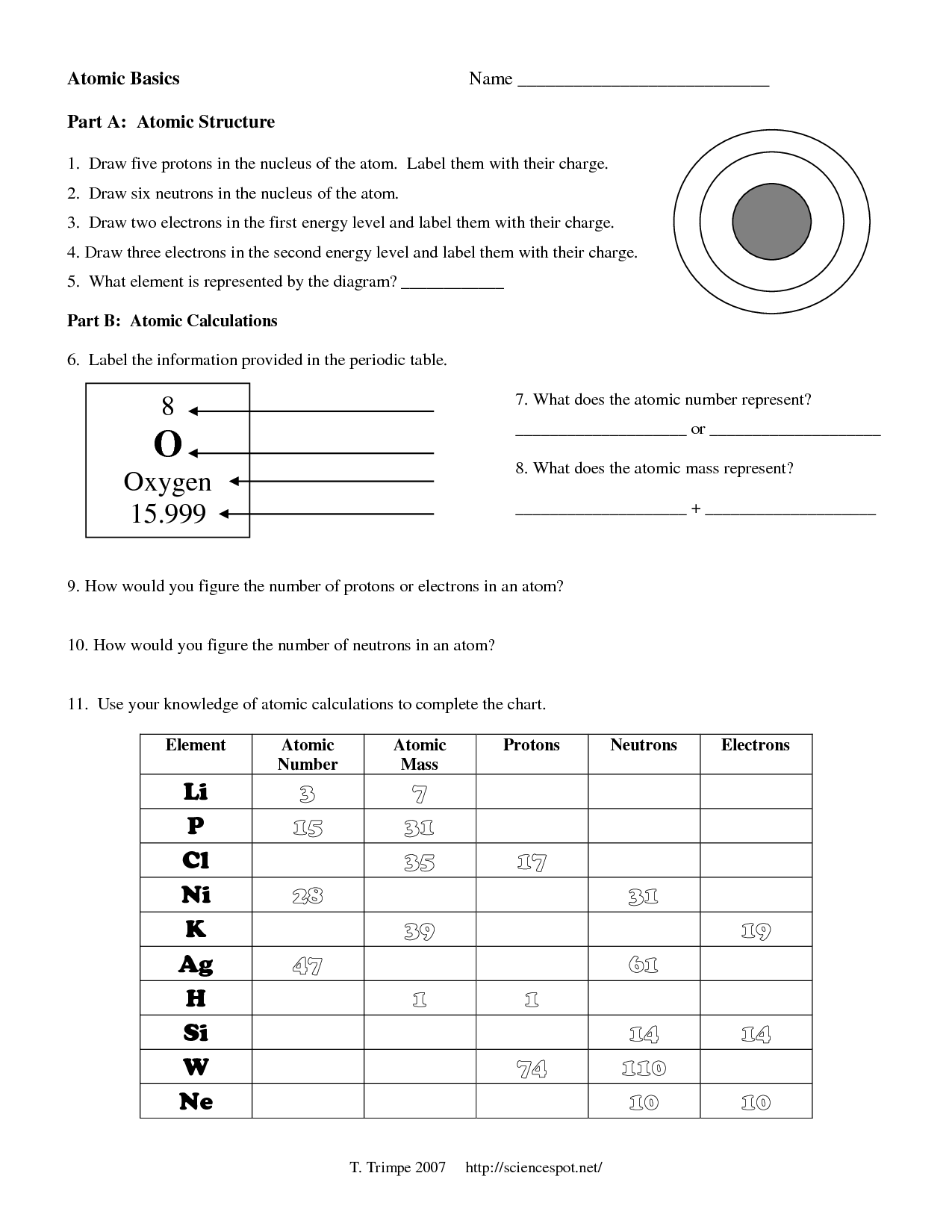
What is an atom?
An atom is the basic unit of matter that consists of a nucleus containing protons and neutrons, surrounded by electrons. It is the smallest particle of an element that retains the properties of that element.
What are the subatomic particles that make up an atom?
An atom is made up of three fundamental subatomic particles: protons, neutrons, and electrons. Protons have a positive charge, neutrons are neutral, and electrons have a negative charge. Protons and neutrons are located in the nucleus of the atom, while electrons orbit around the nucleus in energy levels.
What is the charge of a proton?
The charge of a proton is positive and equal to +1 elementary charge, which is approximately 1.602 x 10^-19 coulombs.
What is the charge of an electron?
The charge of an electron is -1 elementary charge, which is approximately equal to -1.602 x 10^-19 coulombs.
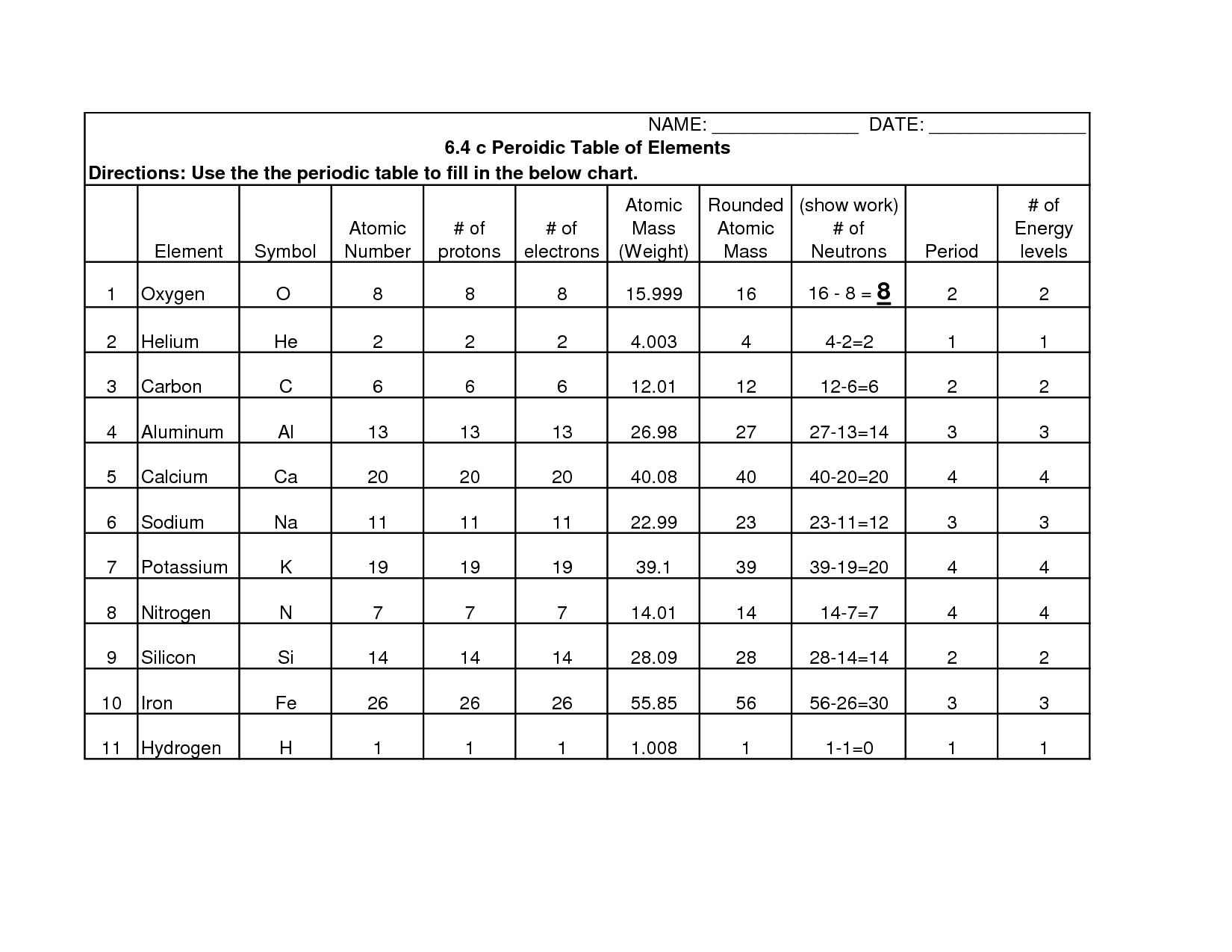
Where are protons and neutrons located within an atom?
Protons and neutrons are located in the nucleus of an atom, which is the central core of the atom. The nucleus makes up the majority of the atom's mass and contains positively charged protons and uncharged neutrons, while negatively charged electrons orbit around the nucleus in specific energy levels.
Where are electrons located within an atom?
Electrons are located outside the nucleus of an atom in specific energy levels or orbitals.
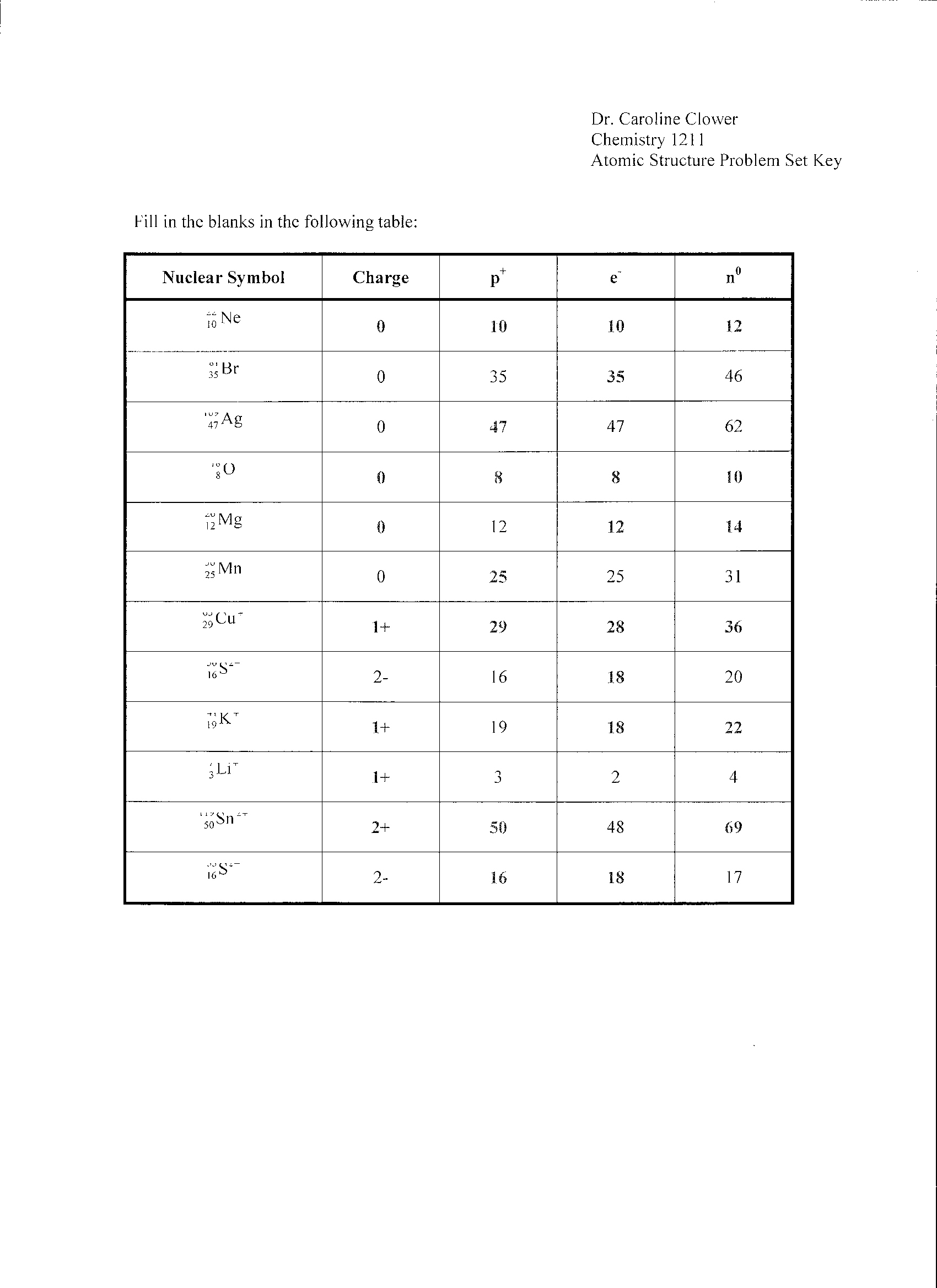
How are the number of protons and electrons related in a neutral atom?
In a neutral atom, the number of protons is equal to the number of electrons. This balance ensures that the positive charge of the protons cancels out the negative charge of the electrons, resulting in a neutral overall charge for the atom.
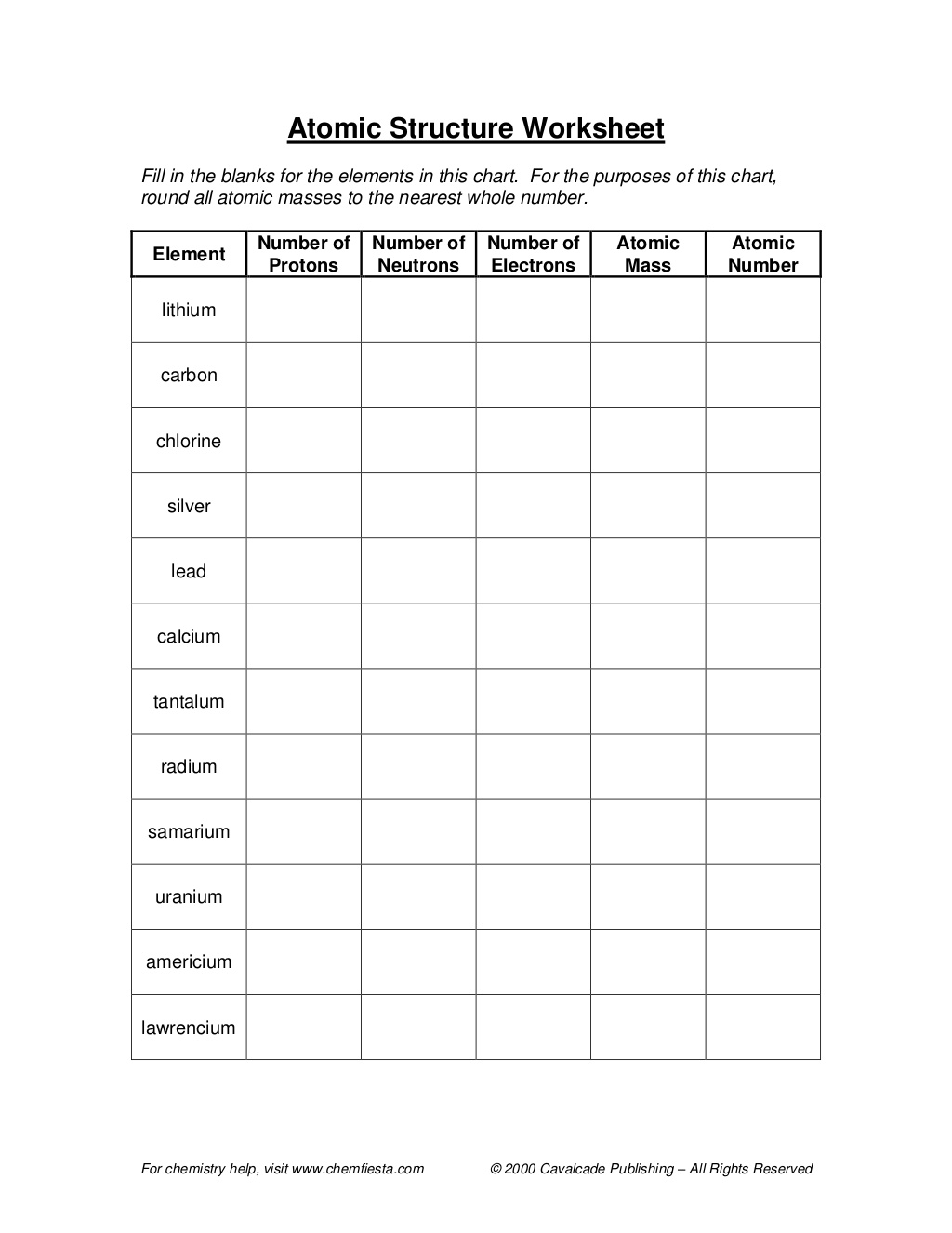
What is an atomic number?
An atomic number is the number of protons found in the nucleus of an atom. It determines the identity of an element and is used to organize the elements on the periodic table. Each element has a unique atomic number that distinguishes it from other elements.
What is an atomic mass?
Atomic mass is the total mass of an atom, which is determined by the sum of the masses of its protons, neutrons, and electrons. It is typically expressed in atomic mass units (amu) or unified atomic mass units (u), with one amu being approximately equal to the mass of a single proton or neutron. The atomic mass of an element is an important factor in determining its properties and behavior in chemical reactions.
How do isotopes differ from each other?
Isotopes are atoms of the same element that contain the same number of protons but different numbers of neutrons. This leads to isotopes having slightly different atomic masses. The differences in atomic masses of isotopes can impact their stability, reactivity, and nuclear properties.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.


























Comments