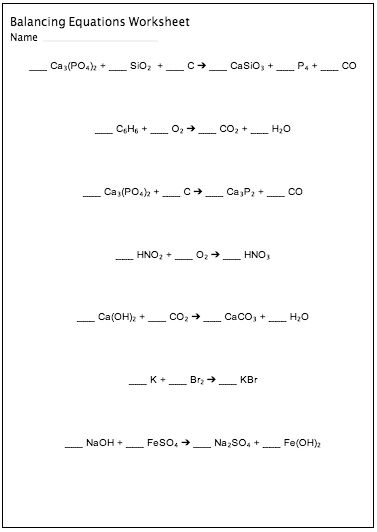
Balancing Equations Worksheet Chemfiesta
The Balancing Equations Worksheet from Chemfiesta is a comprehensive resource designed to help students master the art of balancing chemical equations. With carefully selected examples and step-by-step instructions, this worksheet is perfect for high school chemistry students who are looking to strengthen their understanding of this essential concept.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is the first step in balancing an equation?
The first step in balancing an equation is to write down the unbalanced equation by listing all the reactants and products involved.
Why is it important to balance chemical equations?
Balancing chemical equations is important because it ensures that the law of conservation of mass is obeyed. This law states that matter cannot be created or destroyed in a chemical reaction, only rearranged. By balancing equations, it shows that the number of atoms of each element on both the reactant and product sides are equal, thus demonstrating the conservation of mass. It also helps in determining the correct stoichiometry of the reaction and allows for accurate calculation of quantities involved in the reaction.
How can you determine the coefficients for each compound in a chemical equation?
To determine the coefficients for each compound in a chemical equation, you need to balance the equation by ensuring that the same number of each type of atom is present on both the reactant and product sides. Start by adjusting the coefficients of the compounds in the equation, typically starting with those containing the most complex or abundant elements. Use trial and error to find the smallest whole number coefficients that balance the equation, taking care to adjust one at a time and re-checking the balance after each change.
In balancing equations, what is the difference between a subscript and a coefficient?
A subscript is a number written at the bottom right of a chemical element or compound symbol to indicate the number of atoms of that element in a molecule, while a coefficient is a number placed in front of a chemical formula in a balanced chemical equation to indicate the number of molecules or units of that substance involved in the reaction. In other words, subscripts are used within a chemical formula to show the ratio of atoms in a molecule, whereas coefficients are used in a balanced equation to show the ratio of molecules that react.
How does the law of conservation of mass apply to balanced chemical equations?
The law of conservation of mass applies to balanced chemical equations by stating that the total mass of the reactants must equal the total mass of the products. In other words, during a chemical reaction, atoms are neither created nor destroyed, they are merely rearranged. This principle is reflected in balanced chemical equations, where the number of atoms of each element on the reactant side must be equal to the number of atoms of that element on the product side. This ensures that mass is conserved throughout the reaction.
What is the purpose of adding coefficients to balance equations?
The purpose of adding coefficients to balance equations is to ensure that the conservation of mass and atoms is maintained in a chemical reaction. By adjusting the coefficients, we can make sure that the number of atoms of each element is the same on both sides of the chemical equation, representing a balanced reaction that follows the law of conservation of mass.
Can you balance a chemical equation by changing subscripts?
No, subscripts cannot be changed to balance a chemical equation as they represent the number of atoms of each element in a compound. To balance a chemical equation, coefficients can be added in front of the chemical formulas to ensure that the number of atoms of each element is equal on both sides of the equation.
What are some common techniques used to balance chemical equations?
Some common techniques used to balance chemical equations include: adjusting the coefficients of the molecules, making sure the number of atoms of each element is the same on both sides of the equation, starting with the most complex molecule or element, and using fractions when necessary to ensure balance. It is important to carefully keep track of the atoms and elements during the process to ensure an accurate and balanced equation.
What is the significance of the smallest whole-number coefficients in balanced equations?
The smallest whole-number coefficients in balanced equations represent the simplest ratio in which the reactants combine and the products are formed. The coefficients indicate the number of molecules involved in the chemical reaction, and balancing the equation ensures that mass is conserved and the reaction proceeds correctly. By using the smallest whole numbers, the equation reflects the true stoichiometry of the reaction, making it easier to interpret and work with in chemical calculations.
Are there any exceptions to the rules of balancing chemical equations?
There are no exceptions to the rules of balancing chemical equations as the fundamental principles of conservation of mass and charge must always be followed. The coefficients in front of the chemical formulas are adjusted to ensure that the number of atoms of each element is equal on both sides of the equation. While equations may vary in complexity, the basic rules of balancing remain consistent in all cases.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



































Comments