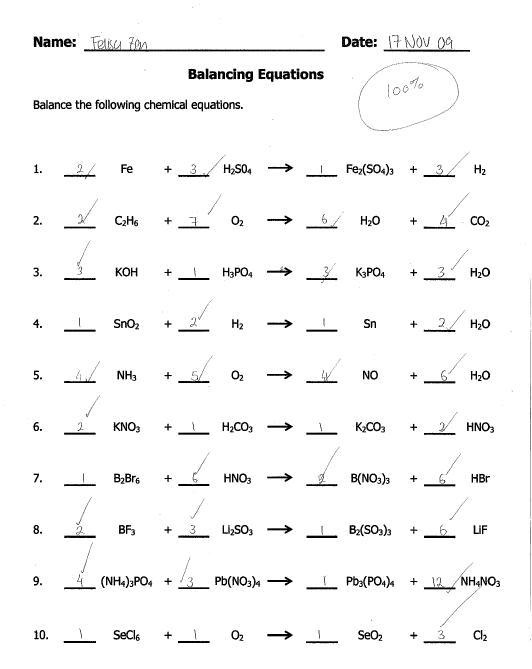
Balancing Equations Practice Worksheet Answers
Are you struggling to understand the concept of balancing equations? Look no further! In this blog post, we will provide you with a practice worksheet that includes answers to help you enhance your understanding and skills in balancing equations. This worksheet is designed for individuals who are new to balancing equations or need extra practice to reinforce their knowledge in this subject.
Table of Images 👆
- Balancing Equations Worksheet Answers
- Balancing Equations Worksheet Answer Key
- Naming Ionic Compounds Worksheet Answer Key
- Balancing Chemical Equations Worksheet Answer Key
- Balancing Chemical Equations Worksheet 1
- Balancing Chemical Equations Worksheet Answers
- Bonding Worksheet Answer Key
- Lewis Dot Structure Worksheet Answers
- Simple Equations with Variables Worksheets
- Variable Expressions and Equations Worksheets
- The New Testament
- Math Algebra 1 Equations
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is the purpose of balancing equations when solving chemical reactions?
Balancing equations in chemical reactions is crucial to ensure that the law of conservation of mass is obeyed. By balancing the equations, the number of atoms on each side of the reaction is equal, meaning that no atoms are lost or gained during the reaction. This allows us to accurately determine the quantities of reactants needed and products formed in a chemical reaction, ensuring its completeness and efficiency.
How do you determine the coefficients of each compound in a chemical equation?
The coefficients of each compound in a chemical equation are determined by balancing the equation, which involves ensuring that the number of atoms of each element is the same on both sides of the equation. This is achieved by adjusting the coefficients of the compounds in the equation until the law of conservation of mass is satisfied. The process involves trial and error, using algebraic methods and chemical intuition to balance the equation.
What are some common methods or strategies used to balance equations?
Some common methods or strategies used to balance equations include adjusting coefficients in front of compounds or elements, ensuring that the number of atoms of each element on both sides of the equation are equal, and using fractions or multiplying all coefficients by a common factor to achieve the desired balance. Another helpful approach is to start by balancing the most complex or uncommon elements first and then working towards the simpler or more common elements in the equation.
Can you give an example of an unbalanced chemical equation and walk through the steps to balance it?
Sure, here is an example of an unbalanced chemical equation: H2 + O2 = H2O. To balance this equation, first count the number of atoms of each element on both the reactant and product sides. In this case, we have 2 hydrogen atoms on the left side and 2 on the right side, but only 1 oxygen on the left side and 2 on the right side. To balance the oxygen atoms, we can add a coefficient of 2 in front of H2O to get 2H2 + O2 = 2H2O. Now, we have 2 hydrogen and 2 oxygen atoms on both sides, making the equation balanced.
What happens if an equation is not balanced?
If an equation is not balanced, it means that the number of atoms on the reactant side is not equal to the number of atoms on the product side. This can lead to inaccurate results in chemical reactions as the Law of Conservation of Mass is violated, where the total mass of the reactants should be equal to the total mass of the products. Balancing an equation is essential to correctly depict the relationship between reactants and products and ensure that mass is conserved in a chemical reaction.
Is it possible to balance an equation without changing the chemical formula of the compounds involved?
Yes, it is possible to balance an equation without changing the chemical formula of the compounds involved. Balancing an equation involves adjusting the coefficients in front of each compound to ensure that the number of each type of atom is the same on both sides of the equation. This can be achieved by changing the coefficients while keeping the actual chemical formula of the compounds unchanged.
Are there any limitations or restrictions when balancing equations?
Yes, there are certain limitations when balancing equations. One key restriction is that you cannot change the subscripts of the elements in the chemical formulas as this would result in changing the identity of the compounds involved. Additionally, the law of conservation of mass must be obeyed, meaning the number of each type of atom must be the same on both sides of the equation. Finally, coefficients can be used to balance equations, but they must be whole numbers to accurately represent the ratios of the reactants and products involved.
How can you identify if an equation is balanced or not?
To identify if an equation is balanced, you need to compare the number of atoms of each element on both sides of the equation. If the same number of each type of atom is present on both sides, the equation is balanced. Balancing an equation involves adjusting the coefficients of reactants and products to ensure that the number of atoms of each element is equal on both sides.
How does balancing equations relate to the law of conservation of mass?
Balancing equations is essential because it ensures that the law of conservation of mass is obeyed. The foundational principle of the law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction, only rearranged. By balancing equations, the same number of atoms of each element on both the reactant and product sides is maintained. This ensures that the total mass of reactants equals the total mass of products, verifying the conservation of mass in the chemical reaction.
Why is it important to accurately balance chemical equations in scientific experiments and calculations?
Accurately balancing chemical equations is crucial in scientific experiments and calculations because it ensures that the law of conservation of mass is being upheld. This law states that mass can neither be created nor destroyed in a chemical reaction, only rearranged. By balancing equations, we can ensure that the same number of atoms of each element are present on both sides of the equation, allowing for precise measurements and calculations to be made. Inaccurate balancing can lead to incorrect results, unreliable data, and ultimately, a flawed understanding of the chemical processes at play.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.

































Comments