Atomic Structure Review Worksheet
Are you a high school student studying chemistry? Do you need a comprehensive review of atomic structure? Look no further, because we have the perfect worksheet for you! This Atomic Structure Review Worksheet will help you understand the key concepts and principles of atoms and their components. With carefully crafted questions and engaging activities, this worksheet will provide the perfect opportunity for you to strengthen your knowledge and mastery of this important subject.
Table of Images 👆
- Atomic Structure Diagram Worksheet
- Lewis Structures Worksheet Answer Key
- Chemistry Worksheet Answer Keys
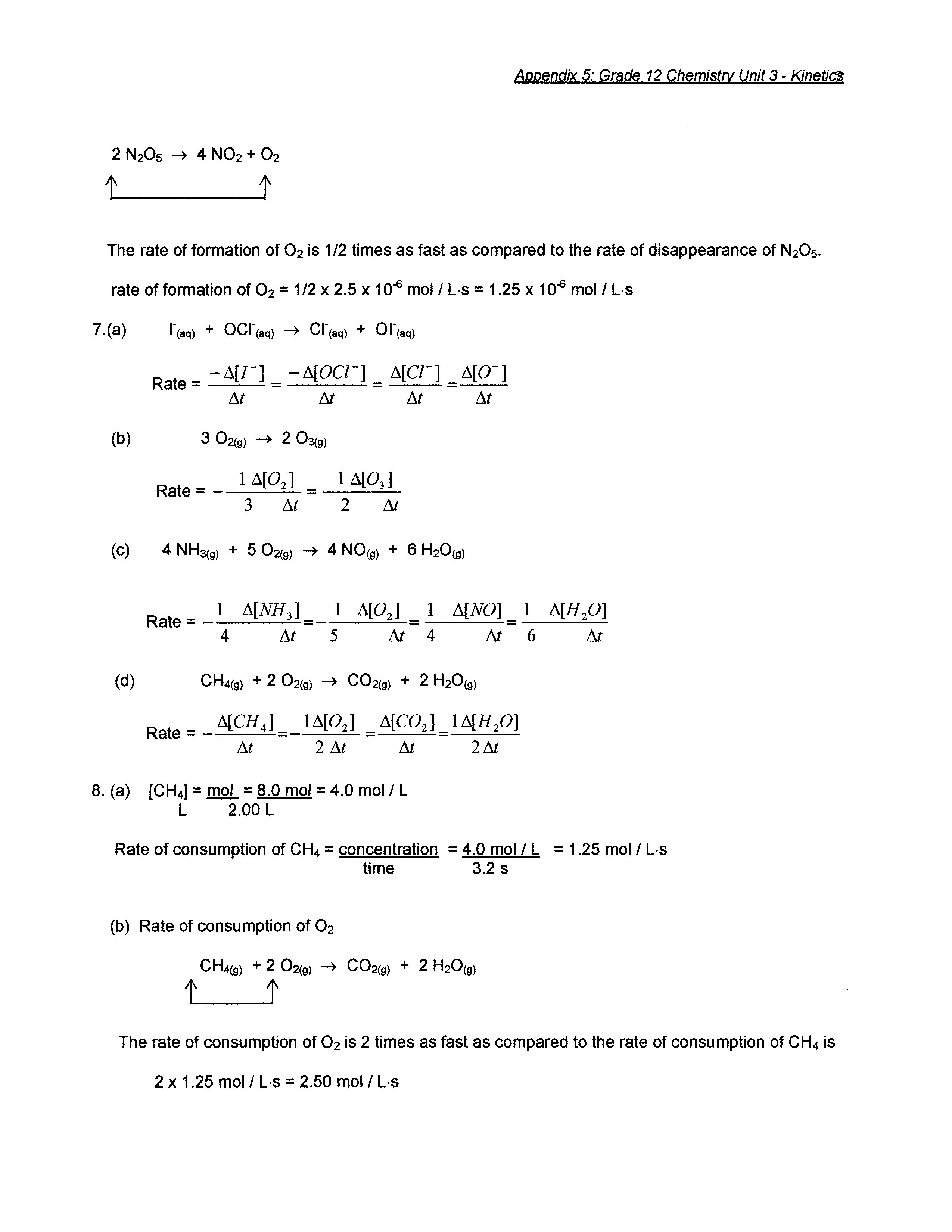
- Chemistry Equations Answer Key Chapter 10 Review
- Lewis Dot Structure Worksheet and Answers
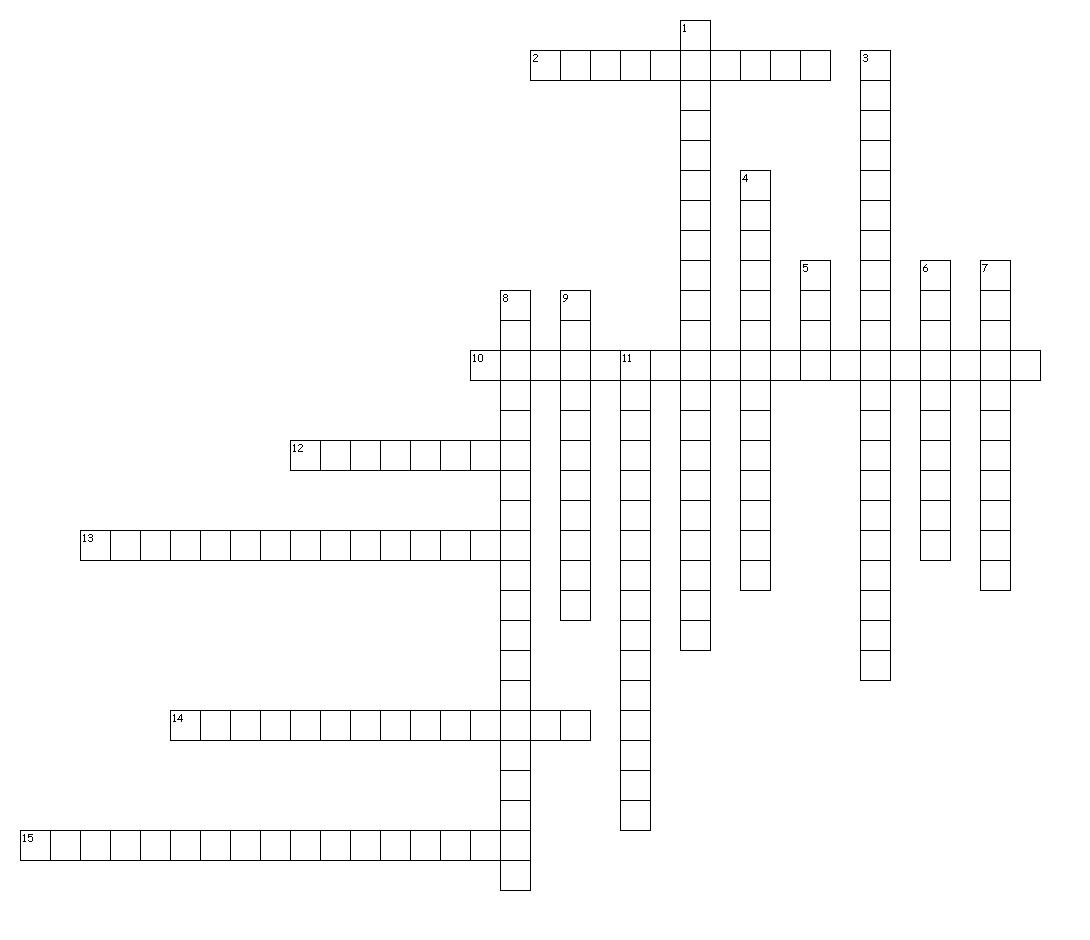
- Chemical Elements Crossword Puzzle Answers
- How to Draw a Bohr Model Diagram
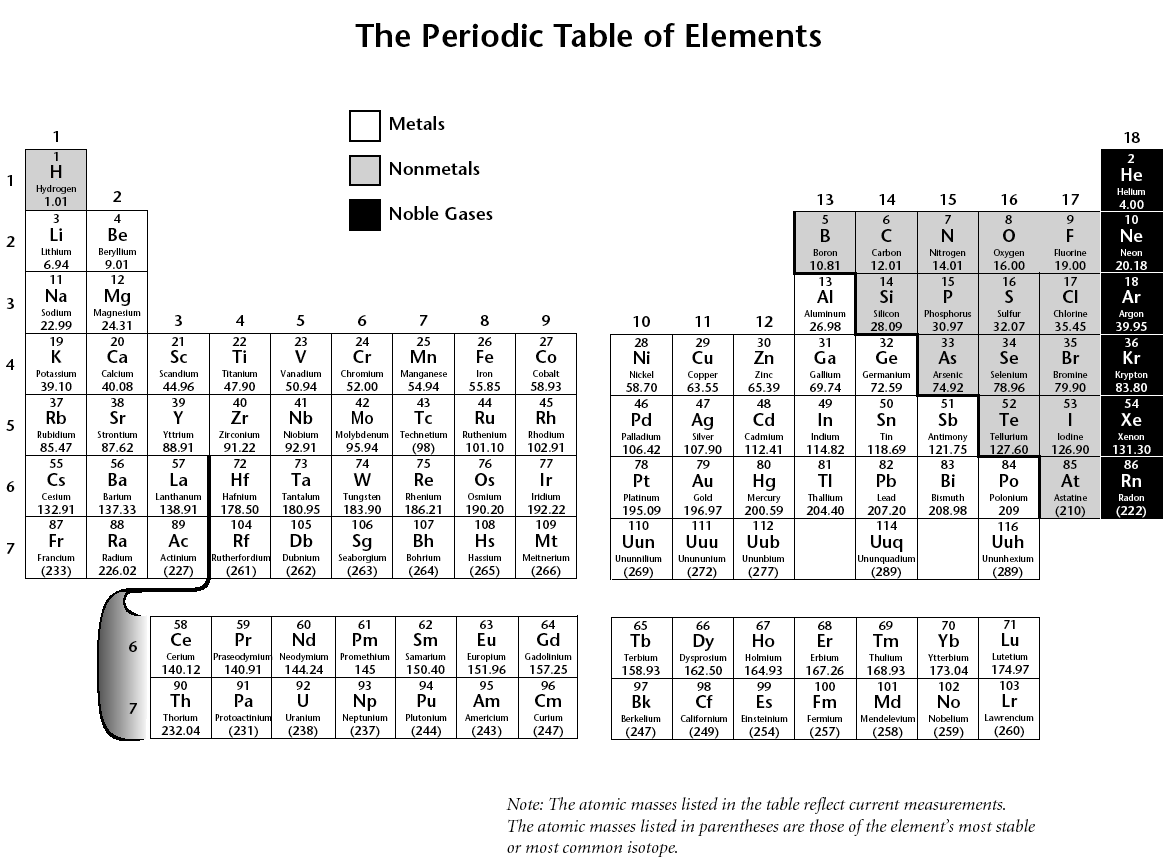
- Periodic Table with Mass Number
- Energy in a Cell Crossword Puzzle Answer Key
- Atomic Structure Foldable
- Solubility Curve Worksheet Answer Key
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is an atom?
An atom is the smallest unit of matter that retains the properties of an element. It consists of a dense nucleus containing positively charged protons and uncharged neutrons, surrounded by negatively charged electrons orbiting in specific energy levels or shells. Atoms combine to form molecules, which in turn make up all substances in the universe.
What are the three subatomic particles that make up an atom, and what are their respective charges?
The three subatomic particles that make up an atom are protons, neutrons, and electrons. Protons have a positive charge, neutrons have no charge (neutral), and electrons have a negative charge.
Where are protons and neutrons located within an atom?
Protons and neutrons are located in the nucleus of an atom. The nucleus is the central core of an atom and contains the majority of the atom's mass, with protons carrying a positive charge and neutrons carrying no charge.
Where are electrons located within an atom?
Electrons are located in specific energy levels or orbitals around the nucleus of an atom. This region is often referred to as the electron cloud or electron shell, and it is where electrons move and interact with each other to form chemical bonds and determine the chemical properties of an element.
What is the atomic number of an element, and what does it represent?
The atomic number of an element represents the number of protons found in the nucleus of an atom of that element. It is a unique identifying characteristic of an element and determines its place on the periodic table. The atomic number also dictates the chemical properties of the element, as it defines the number of electrons in a neutral atom.
What is the mass number of an atom, and what does it represent?
The mass number of an atom is the total number of protons and neutrons in its nucleus. It represents the atomic mass or weight of the atom and is a key characteristic used to identify different isotopes of an element. The mass number, along with the atomic number (which is the number of protons in the nucleus), determines the identity of the element and its position on the periodic table.
What is an isotope?
An isotope is a variant of a chemical element that has the same number of protons in its nucleus but a different number of neutrons, leading to variations in atomic weight. This can result in isotopes having different physical properties, such as stability and radioactivity.
What is an ion?
An ion is an atom or molecule that has gained or lost one or more electrons, giving it a positive or negative electrical charge. This charge makes ions reactive and able to participate in chemical reactions and form compounds.
What is the difference between an element and a compound?
An element is a pure substance made up of only one type of atom, such as oxygen or iron, while a compound is a substance composed of two or more elements that are chemically bonded together, such as water (H2O) or sodium chloride (NaCl). Elements cannot be broken down into simpler substances through chemical reactions, whereas compounds can be broken down into their constituent elements.
What did Rutherford's gold foil experiment demonstrate about the structure of an atom?
Rutherford's gold foil experiment demonstrated that the atom has a small, dense, positively charged nucleus at its center with electrons surrounding it in a mostly empty space. This overturned the earlier model of the atom as a solid, indivisible sphere, highlighting the presence of a nucleus and the mostly empty space within an atom.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.








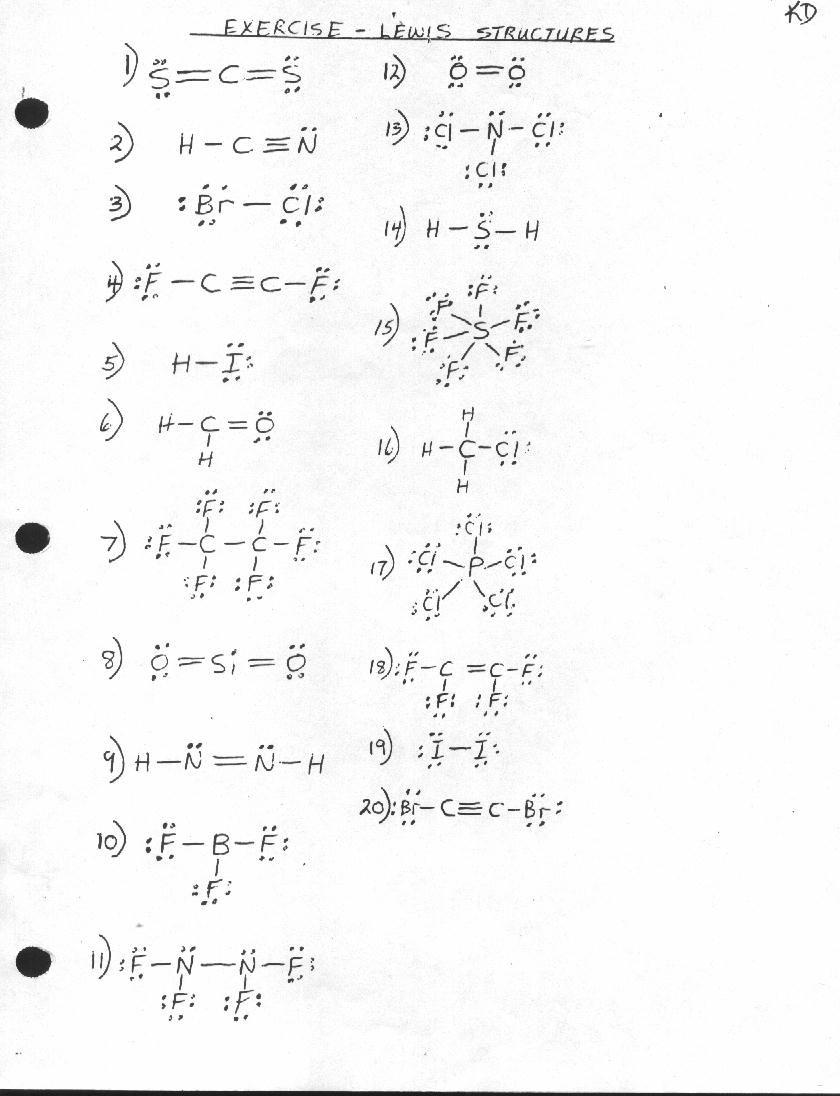


















Comments