Atomic Structure Coloring Worksheet
The Atomic Structure Coloring Worksheet is a comprehensive tool designed to help middle and high school students understand the complex world of atoms and their subatomic particles. This engaging and educational worksheet is perfect for science teachers and homeschooling parents who want to provide their students with a hands-on, visually stimulating learning experience.
Table of Images 👆
- Atomic Structure Worksheet Middle School
- Modern Atomic Theory Worksheet
- Bohr Model Worksheet Answers
- Atomic Structure of an Atom Worksheet
- Chemistry Atomic Structure Worksheet Answers
- Periodic Table Atomic Number Worksheet
- Atomic Structure of Atoms Worksheets
- Atomic Structure Practice Worksheet
- Atomic Structure Worksheet and Periodic Table
- Counting Atoms Worksheet Answer Key
- Bohr Atomic Model Worksheet
- Review Atomic Structure and Periodic Table
- Cell Theory Timeline Worksheet
- Motion Worksheets Middle School
- Electron Configuration Practice Worksheet Answer Key
- Music Rhythm Counting Worksheets
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
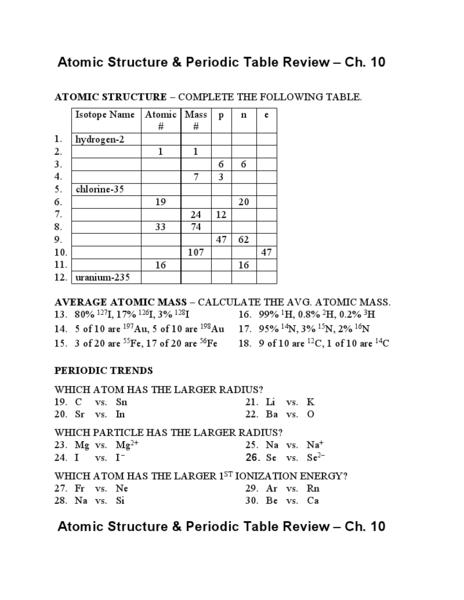
What is the atomic structure?
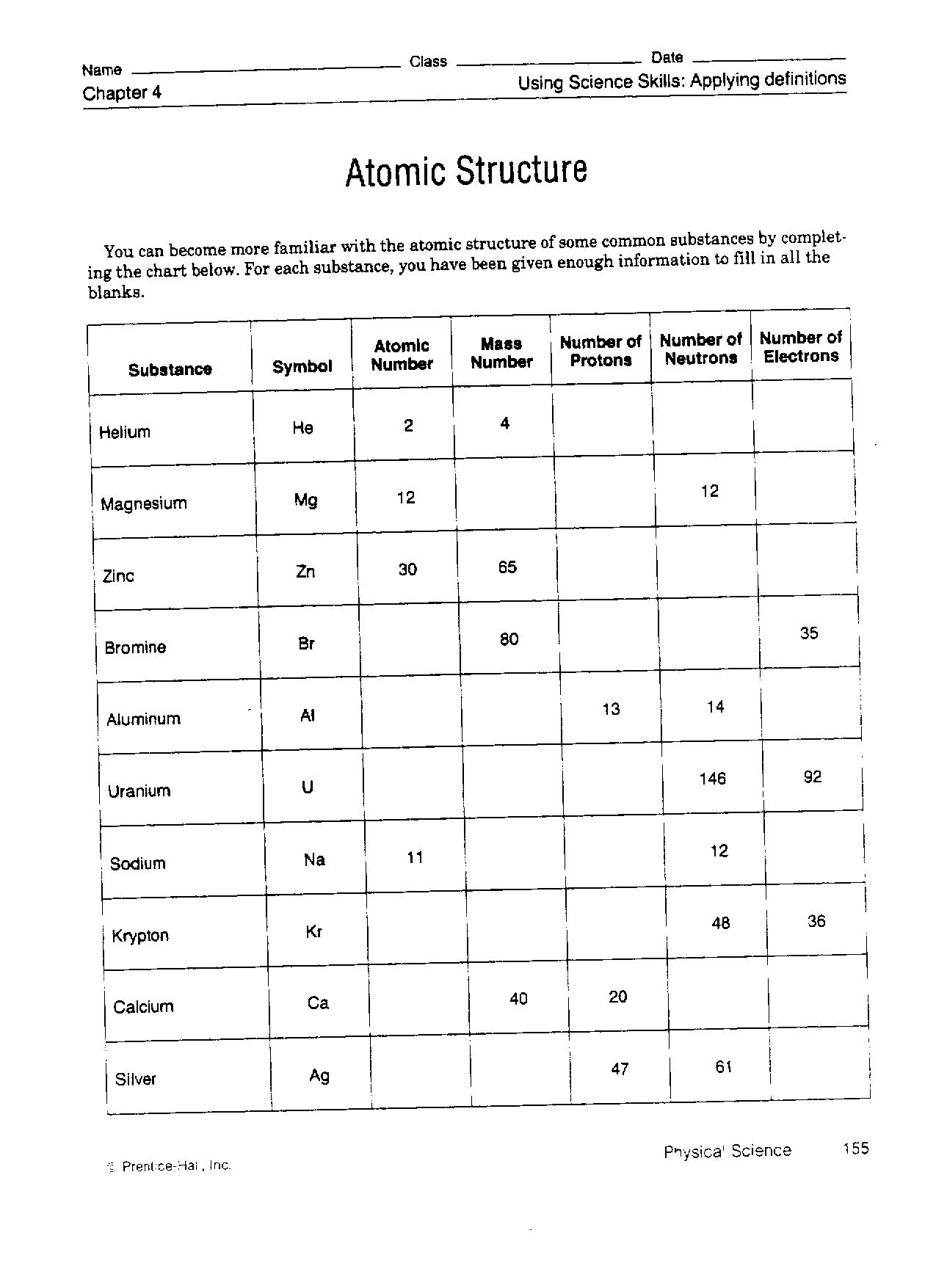
The atomic structure refers to the composition and arrangement of particles within an atom. At the center of the atom is a nucleus containing protons and neutrons, while electrons orbit around the nucleus in specific energy levels or shells. Protons are positively charged, neutrons are neutral, and electrons are negatively charged. The number of protons determines the element's atomic number, and the arrangement of electrons determines the atom's properties and how it interacts with other atoms.
What are atoms made up of?
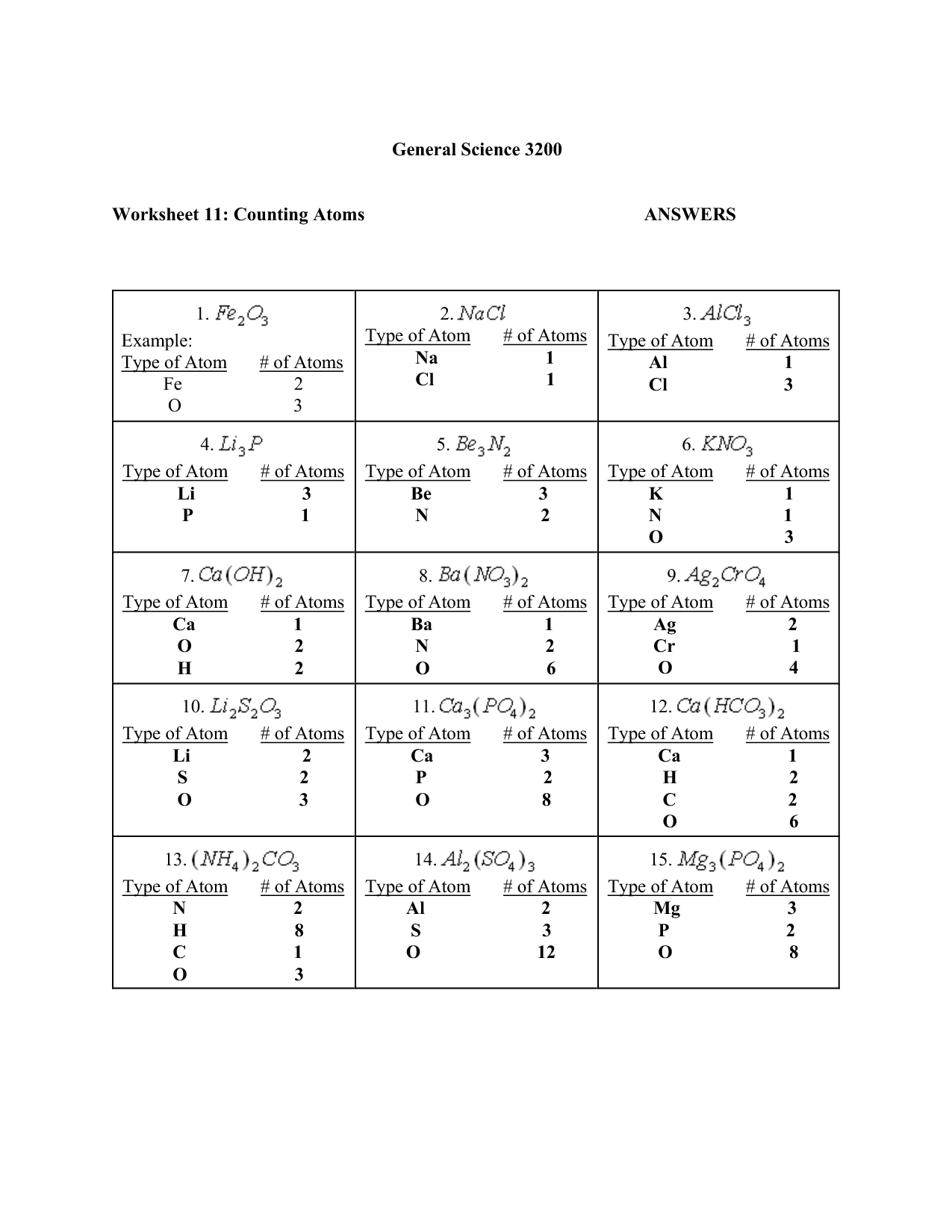
Atoms are made up of subatomic particles, namely protons, neutrons, and electrons. Protons and neutrons are located in the nucleus of the atom, while electrons orbit around the nucleus. These subatomic particles have specific properties and charges that determine the behavior and characteristics of atoms.
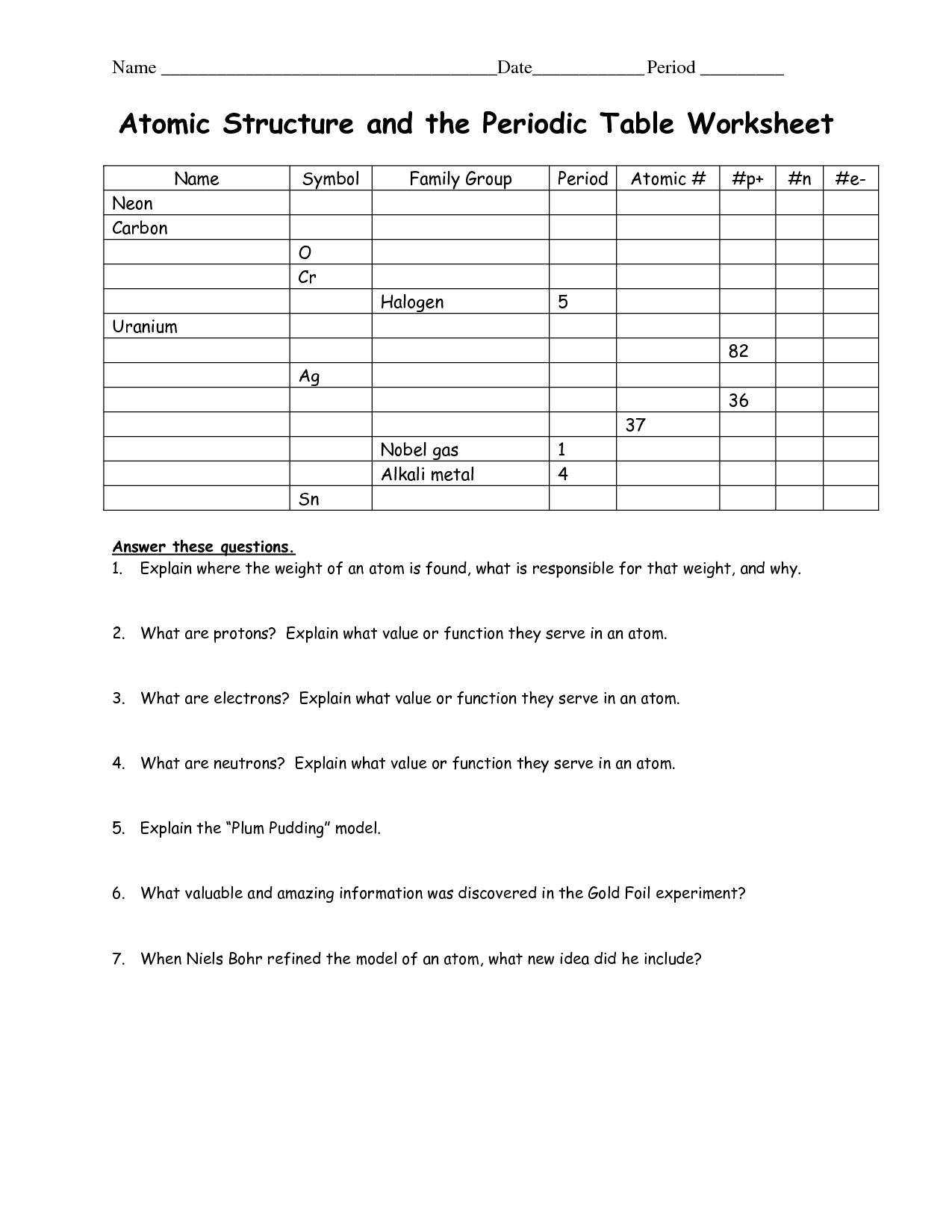
What is the function of protons?
Protons have a positive charge and are found in the nucleus of an atom. Their main function is to contribute to the atomic number of an element, which determines its identity on the periodic table. Additionally, protons play a crucial role in holding the nucleus together through the strong nuclear force, preventing it from breaking apart due to repulsion between positively charged protons.
Describe the charge of electrons.
Electrons are negatively charged particles that are fundamental components of atoms. They carry a unit negative charge of -1.6 x 10^-19 coulombs and are responsible for generating electricity, conducting heat, and forming chemical bonds in atoms and molecules.
Explain the location of neutrons in an atom.
Neutrons are located in the nucleus of an atom, along with protons. The nucleus is the central part of the atom that contains most of the atom's mass and is positively charged. Neutrons have no electric charge, unlike protons which are positively charged, and they help stabilize the nucleus by providing additional nuclear binding energy.
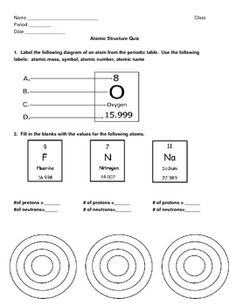
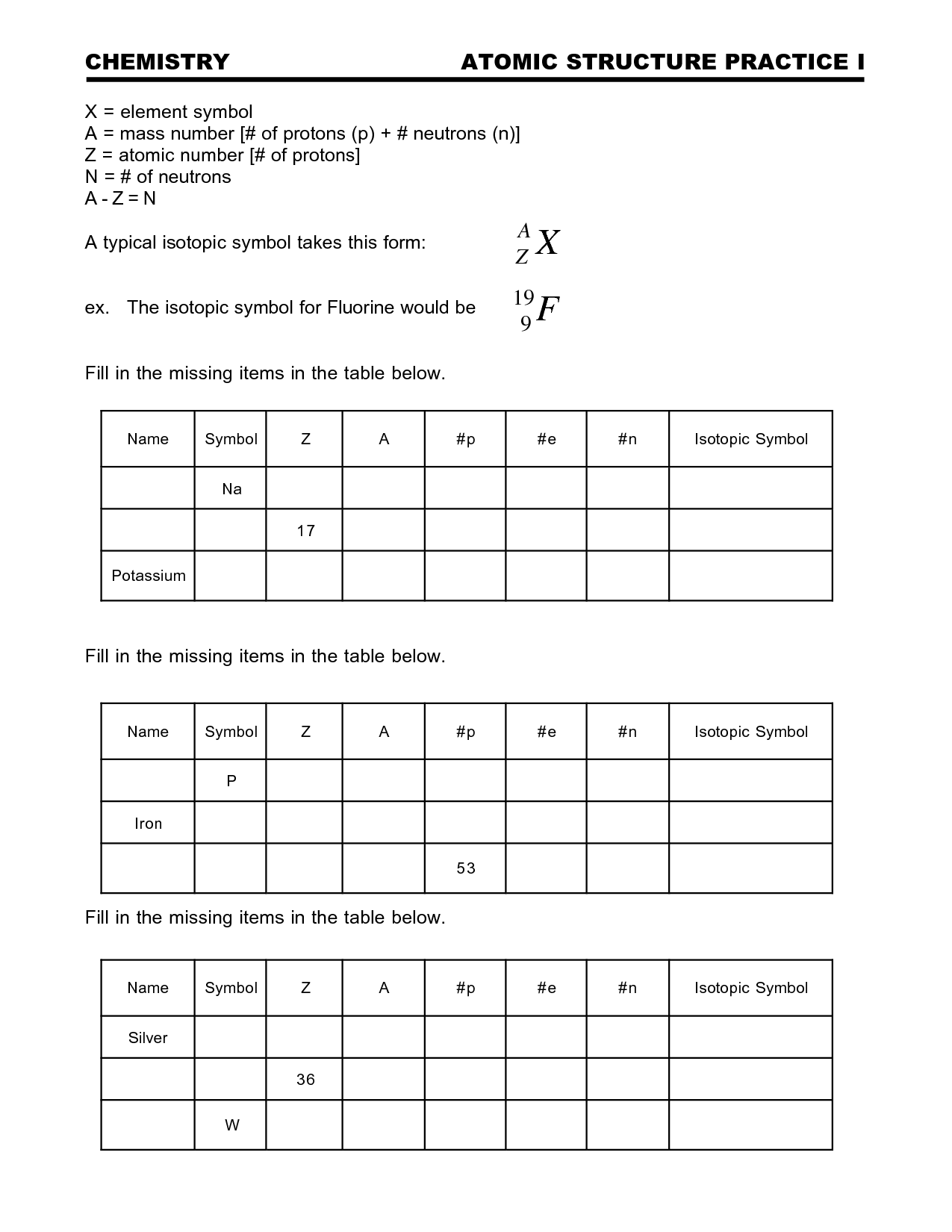
What is the atomic number?
The atomic number of an element is the number of protons found in the nucleus of its atom, which uniquely identifies an element.
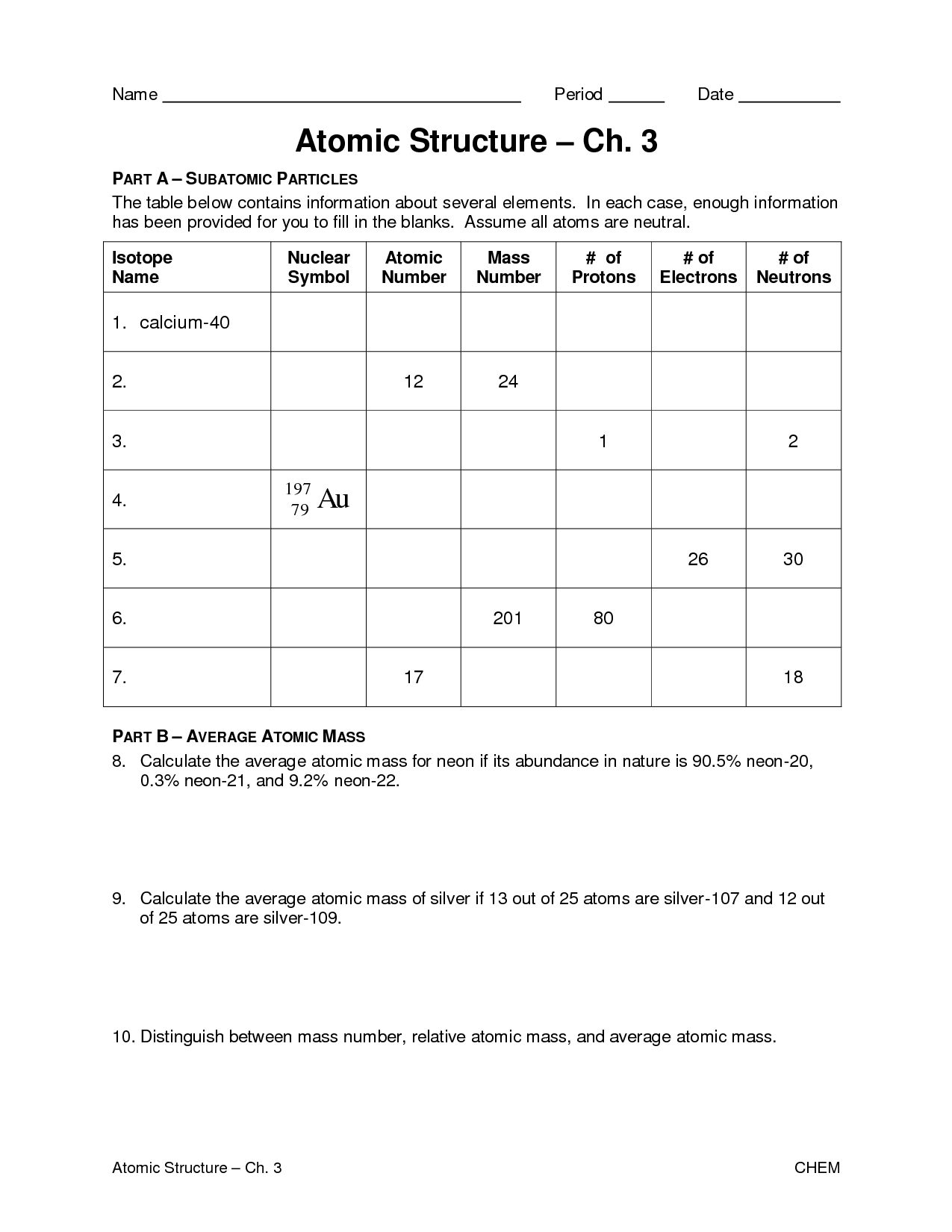
What does the atomic mass represent?
The atomic mass represents the average mass of an atom of an element, taking into account the different isotopes and their relative abundance in nature. It is measured in atomic mass units (amu) and is a weighted average of the masses of the different isotopes of an element.
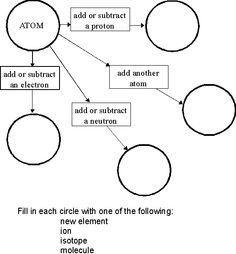
How do isotopes differ from each other?
Isotopes differ from each other based on the number of neutrons in their atomic nuclei. While isotopes of the same element have the same number of protons and electrons, they can have varying numbers of neutrons. This difference in neutron number leads to variations in atomic mass between isotopes of the same element.
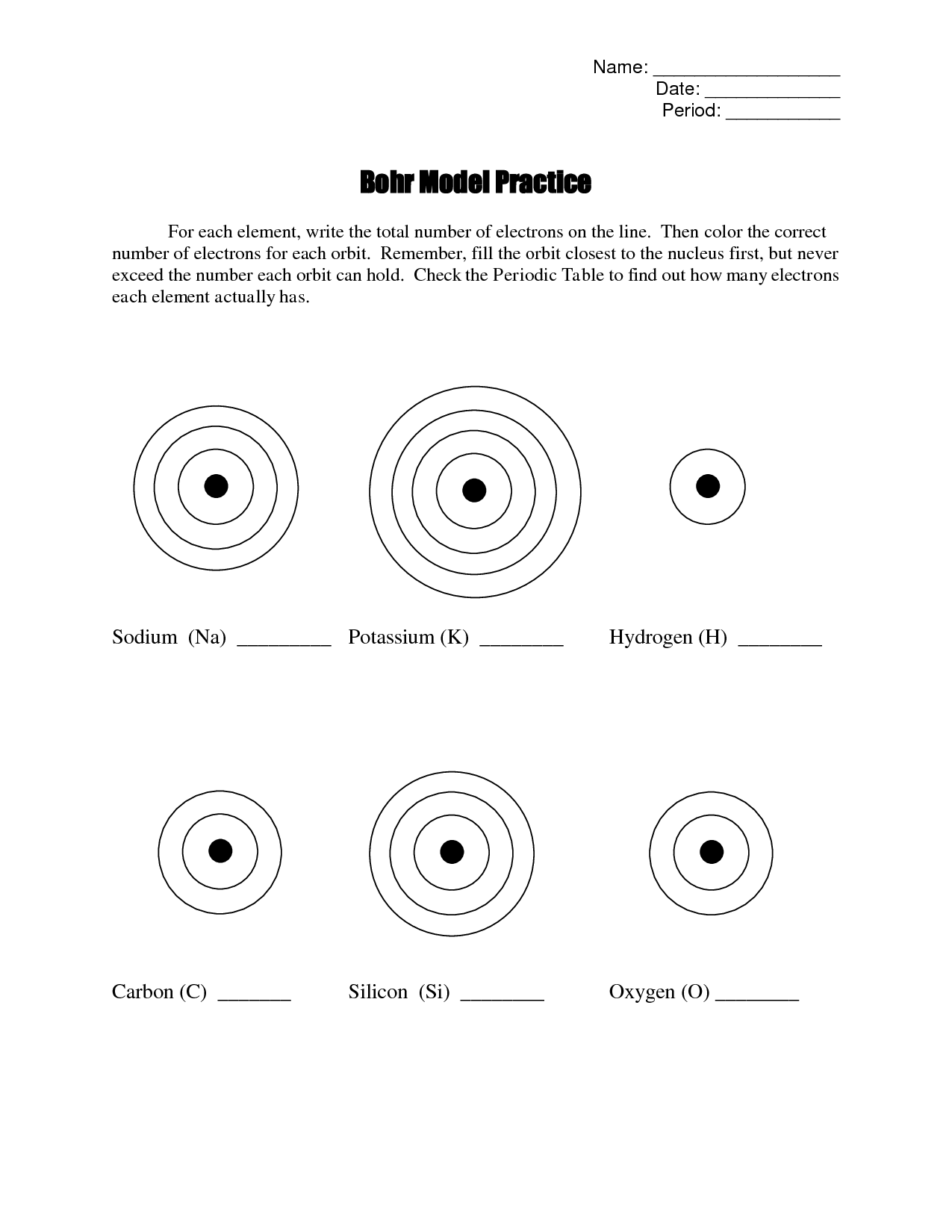
Describe the energy levels or shells in an atom.
Energy levels or shells in an atom are regions where electrons are most likely to be found. These shells are designated by the principal quantum number (n), starting from the closest to the nucleus (n=1) and increasing outward. Each shell can hold a specific number of electrons, with higher energy levels being further from the nucleus and holding more electrons. Electrons fill the shells in order of increasing energy, with the innermost shell being filled first before moving to the next shell. The energy levels determine the behavior and properties of atoms, as well as how they form chemical bonds with other atoms.
What determines the chemical properties of an element?
The chemical properties of an element are determined by its atomic structure, specifically the arrangement of electrons in its outermost energy level. This includes factors such as the number of electrons, the distribution of electrons within the energy levels, and the ability of the atom to gain, lose, or share electrons to achieve a stable configuration. These factors influence an element's reactivity, ability to form chemical bonds, and ultimately its chemical behavior.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.























Comments