Science Worksheets Balancing Equations
Science worksheets are a valuable resource for students who want to strengthen their understanding of balancing equations. Whether you are a middle school student just beginning to learn about chemical reactions or a high school student honing your skills, worksheets provide a structured approach to mastering this fundamental concept.
Table of Images 👆
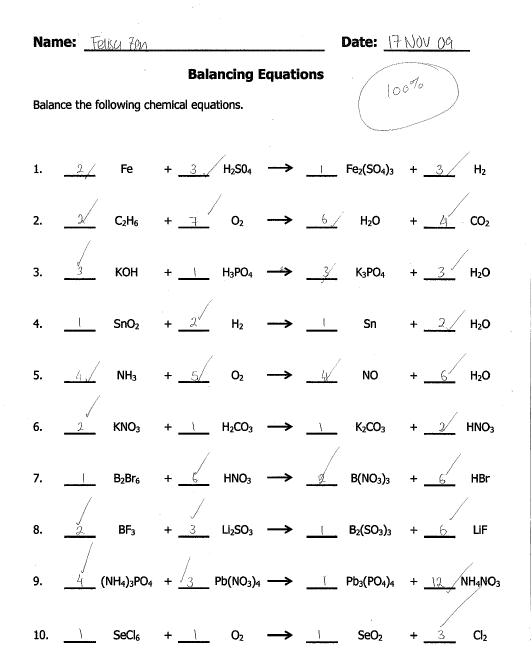
- Balancing Equations Worksheet Answers
- Balancing Chemical Equations Worksheet Answers
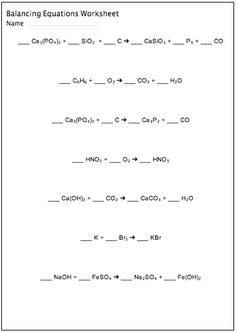
- Balancing Chemical Equations Worksheet
- 8th Grade Earth Science Worksheets
- Balancing Chemical Equations Worksheet 1 Answers
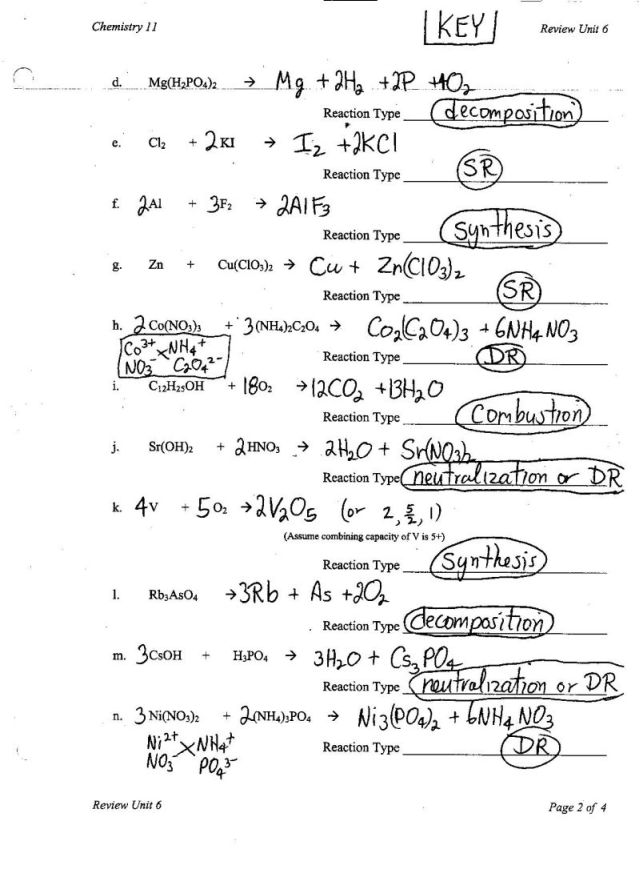
- Balancing Chemical Equations Worksheet Answer Key
- Charles Law Worksheet Answers
- Types of Chemical Reactions Worksheet Answer Key
- Gas Stoichiometry Worksheet Answer Key
- Chemical Formula for Polyatomic Ions
More Science Worksheets
6 Grade Science WorksheetsScience Heat Energy Worksheets with Answer
Science Worksheets Light and Sound
7th Grade Science Cells Worksheets
Worksheets Life Science Vocabulary
8th Grade Science Scientific Method Worksheet
Science Worksheets All Cells
What is a balanced equation in chemistry?
A balanced equation in chemistry is an equation where the number of atoms for each element is the same on both the reactant and product sides. This ensures that mass is conserved in the chemical reaction, with the same amount of each element present before and after the reaction occurs. Balancing the equation involves adjusting the coefficients in front of the chemical formulas to achieve this equality.
How do you balance a chemical equation?
To balance a chemical equation, you need to ensure that the number of atoms of each element on the reactant side is equal to the number of atoms of each element on the product side. Start by adjusting the coefficients in front of each molecule so that the number of atoms of each element is the same on both sides of the equation. Remember that you can only change coefficients, not subscripts, to balance the equation. Keep adjusting the coefficients until all elements are balanced.
Why is it important to balance equations in science?
Balancing equations in science is crucial because it ensures that the law of conservation of mass is followed, meaning that there is an equal number of atoms of each element on both sides of the equation. This helps maintain the integrity and accuracy of scientific calculations and predictions, as well as provides valuable information about the reactants and products involved in a chemical reaction..SimpleDateFormat: 72cf415cf1e0b5a1dc5e57e2e5f074b6,2021-10-06T12:14:12.308716Z
What are reactants and products in a chemical equation?
Reactants are the substances that are present at the start of a chemical reaction and undergo a change, while products are the substances formed as a result of that reaction. In a chemical equation, reactants are written on the left side of the equation and products are written on the right side, showing the transformation that occurs during the reaction.
What is the purpose of coefficients in a balanced equation?
Coefficients in a balanced equation are used to ensure that the number of each type of atom on the reactant side is equal to the number of each type of atom on the product side. By adjusting the coefficients, we can balance the equation and show the conservation of mass in a chemical reaction.
Can you give an example of a balanced chemical equation?
Certainly! An example of a balanced chemical equation is the reaction between hydrogen gas (H2) and oxygen gas (O2) to form water (H2O): 2H2 + O2 -> 2H2O. In this equation, the number of atoms of each element is the same on both sides, ensuring that mass is conserved in the reaction.
What are the principles or rules followed when balancing equations?
When balancing equations, the principles or rules followed include ensuring that the number of atoms of each element on the reactant side is equal to the number of atoms of the same element on the product side, adjusting coefficients (not subscripts) to achieve this balance, keeping the formula of each reactant and product unchanged, and using the lowest whole number coefficients. Additionally, it's important to start by balancing the more complex or least abundant elements first and to double-check the balance by counting the total number of atoms on both sides of the equation.
How does balancing equations help in understanding chemical reactions?
Balancing equations helps in understanding chemical reactions by ensuring that the number of atoms of each element involved in the reaction is the same on both the reactant and product sides. This conservation of mass principle allows us to clearly see how atoms rearrange to form new substances in a reaction. Balancing equations also helps in determining the stoichiometry of a reaction, which is crucial for predicting the amounts of reactants needed and products formed. Overall, balancing equations is a fundamental aspect of understanding and interpreting chemical reactions accurately.
How does balancing equations play a role in stoichiometry calculations?
Balancing equations is crucial in stoichiometry calculations as it ensures that the equation represents the correct ratio of reactants and products involved in a chemical reaction. Balanced equations allow for accurate determination of the mole ratios between reactants and products, which are essential for calculating the quantities of substances involved in a reaction, such as mass, volume, or moles. Without a balanced equation, stoichiometry calculations would be inaccurate and unreliable, leading to incorrect conclusions about the reaction.
What are some common mistakes made when balancing chemical equations?
Some common mistakes made when balancing chemical equations include forgetting to adjust coefficients, not double-checking calculations, ignoring polyatomic ions, incorrectly balancing charges, and forgetting to balance the equation overall. It's important to carefully examine each element and ensure that the number of atoms on both sides of the equation is balanced to accurately represent the chemical reaction.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



























Comments