Chemistry Metrics and Measurements Worksheet
Are you struggling to grasp the concepts of metrics and measurements in chemistry? Look no further than our comprehensive Chemistry Metrics and Measurements Worksheet! Designed specifically for students who need extra practice in this topic, this worksheet will provide you with the perfect opportunity to solidify your understanding of the entity and subject.
Table of Images 👆
More Chemistry Worksheets
What is the purpose of measuring in chemistry?
The purpose of measuring in chemistry is to quantify substances and their properties in order to understand and control chemical reactions, determine the composition of materials, and analyze the behavior of chemical systems. Measurement allows chemists to make accurate observations, collect data, and draw meaningful conclusions that help in the development of new materials, medicines, and technologies, as well as in solving environmental and health-related issues.
What is the SI unit for mass?
The SI unit for mass is the kilogram (kg).
How is temperature measured in chemistry?
Temperature in chemistry is typically measured using a thermometer, which is designed to detect the degree of hotness or coldness of a substance. Thermometers can be filled with a variety of liquids such as mercury or alcohol that expand or contract in response to temperature changes, causing the level of the liquid to rise or fall in a calibrated scale. Modern thermometers may also use electronic sensors to measure temperature and display the results digitally.
What is the difference between precision and accuracy?
Precision refers to the closeness of two or more measurements to each other, indicating consistency and repeatability. On the other hand, accuracy refers to how close a measured value is to the true or accepted value, reflecting the lack of systematic error in the measurement process. In simpler terms, precision is about reproducibility, while accuracy is about truthfulness.
How is volume typically measured in the laboratory?
Volume is typically measured in the laboratory using graduated cylinders, pipettes, burettes, or volumetric flasks. These instruments are designed to accurately measure liquid volumes in various capacities, depending on the level of precision required for the experiment or analysis being conducted.
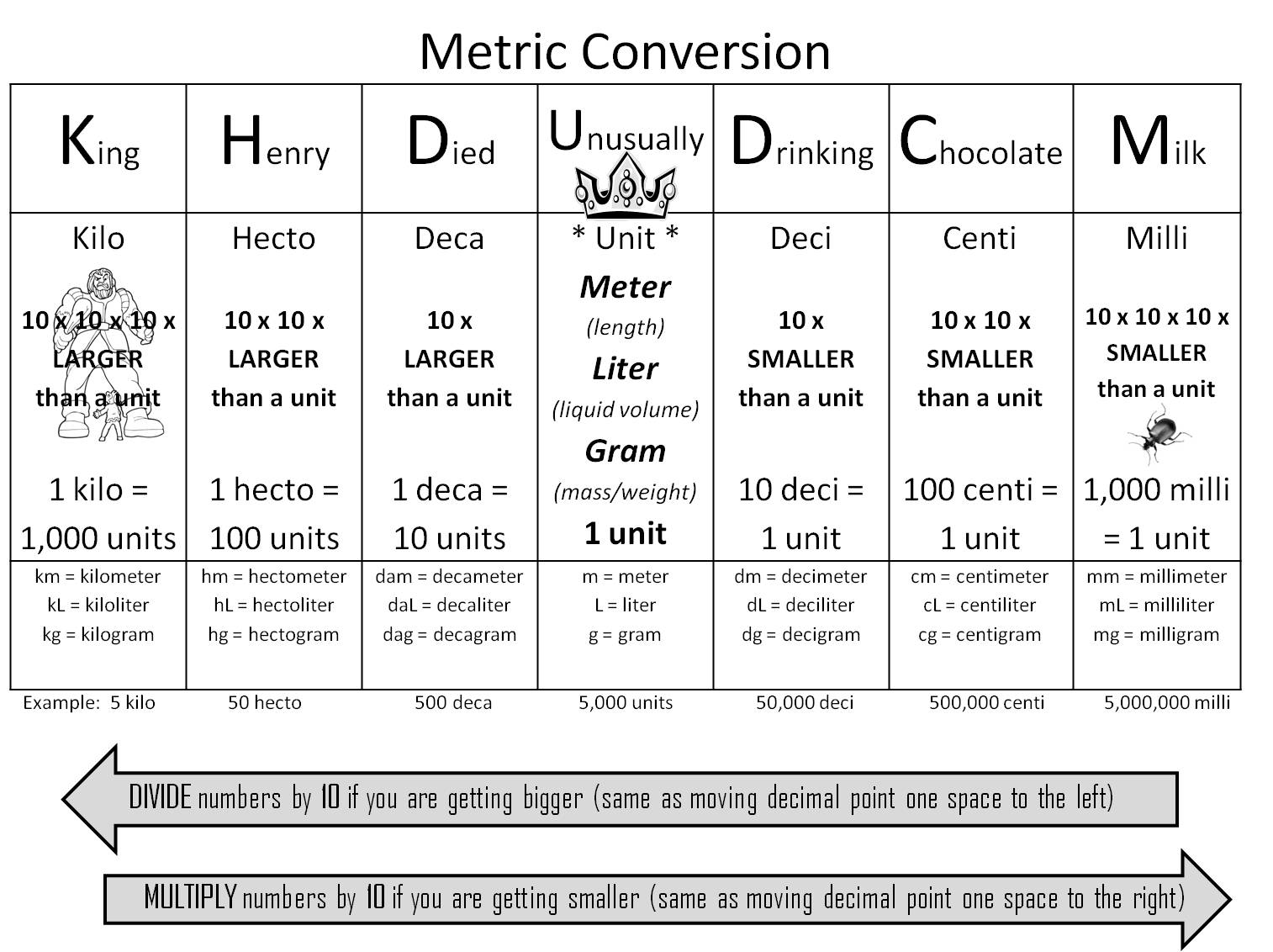
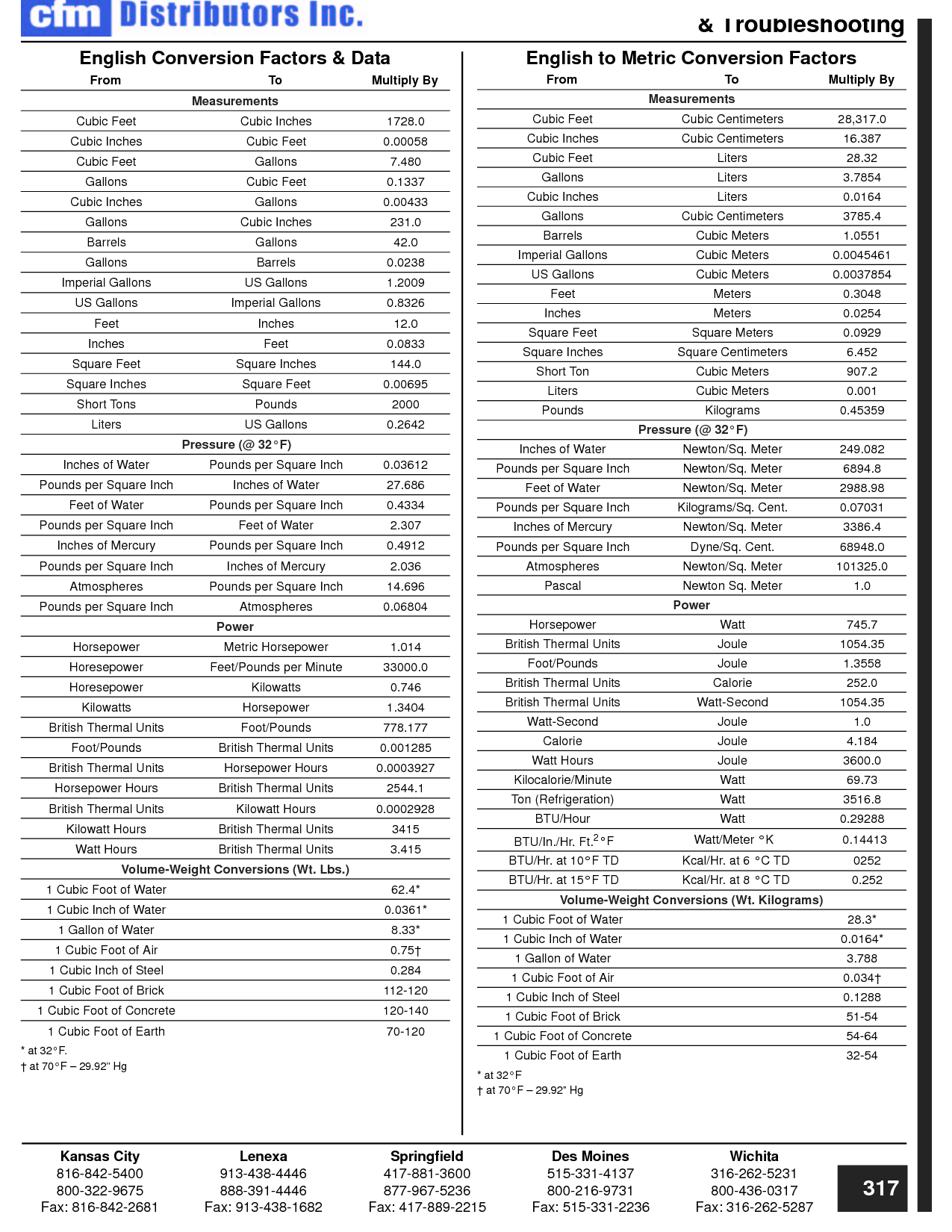
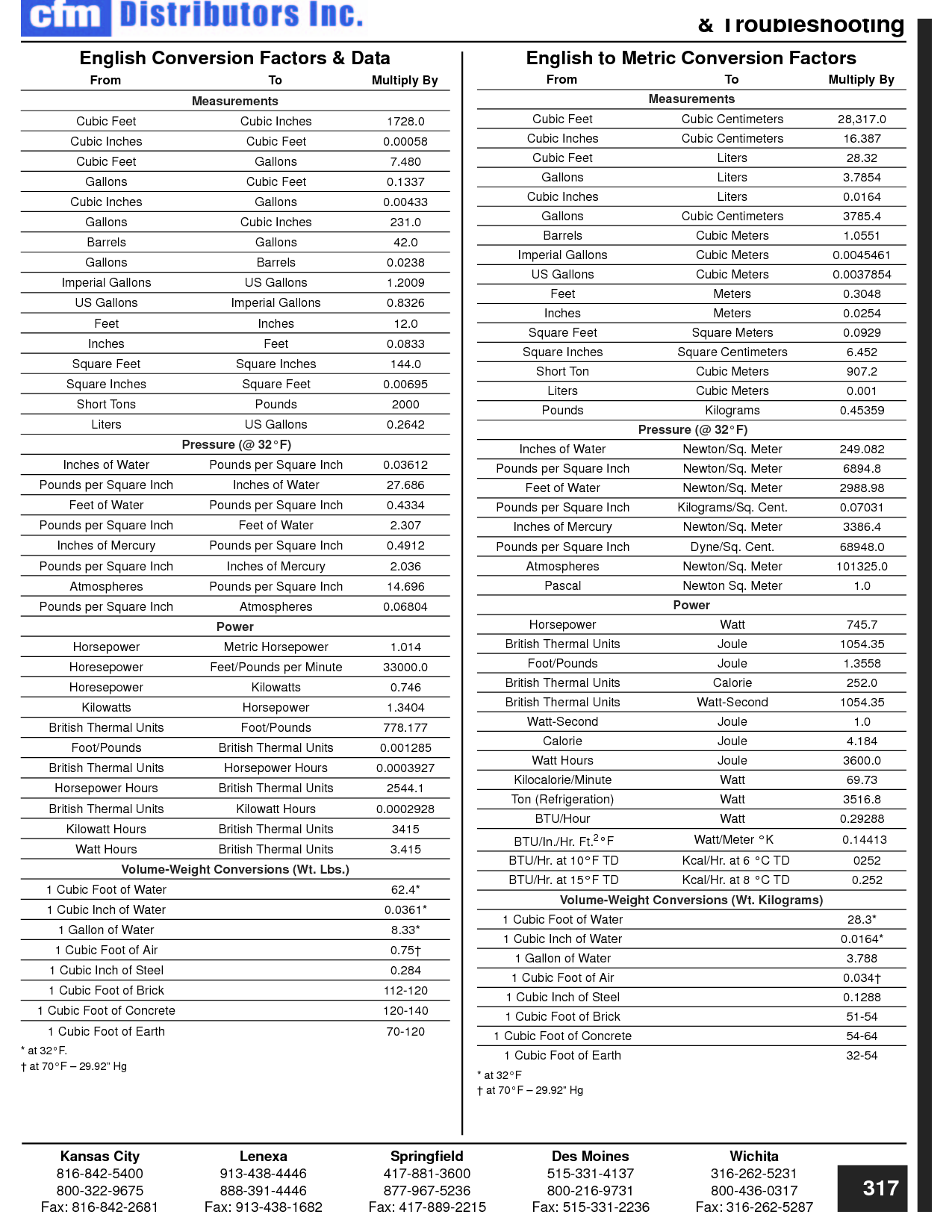
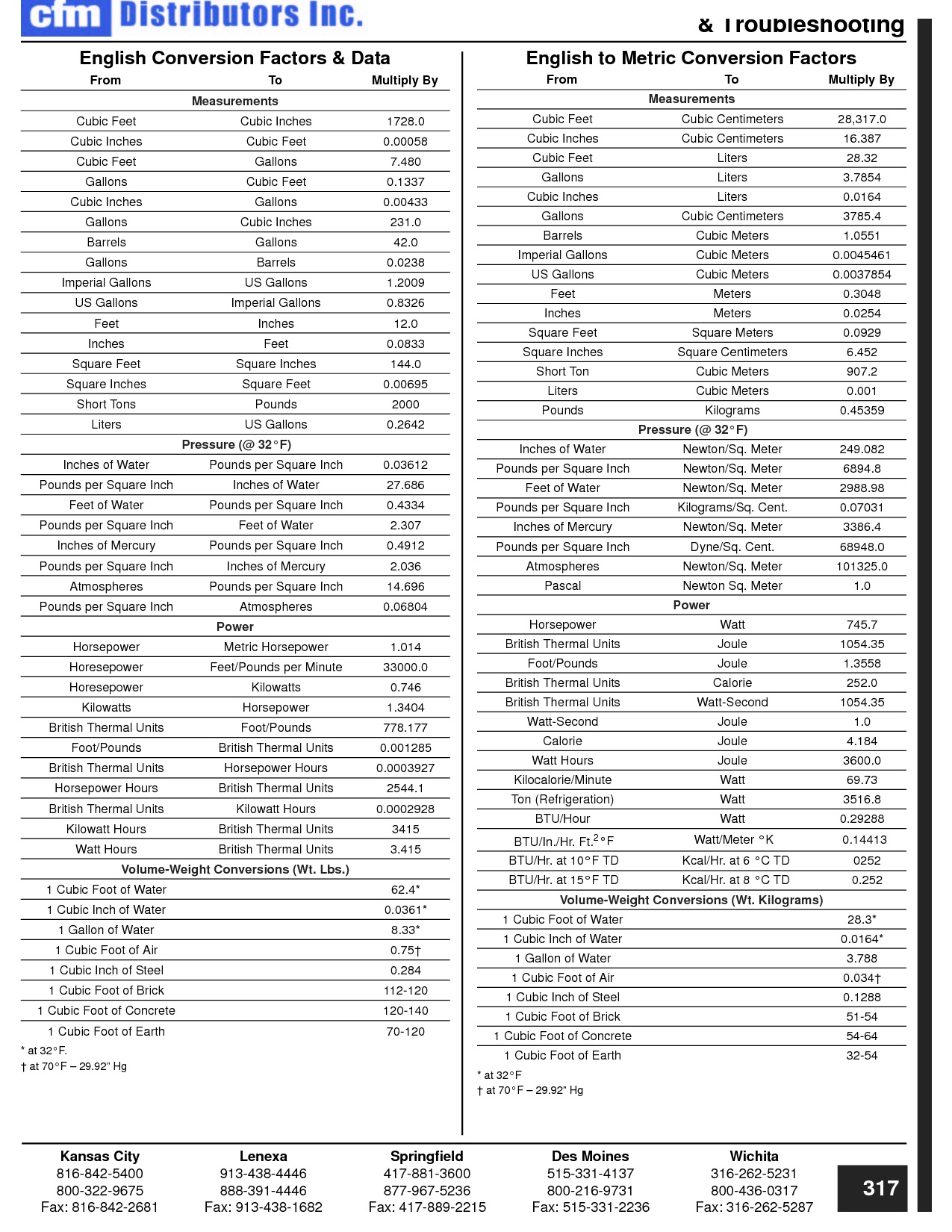
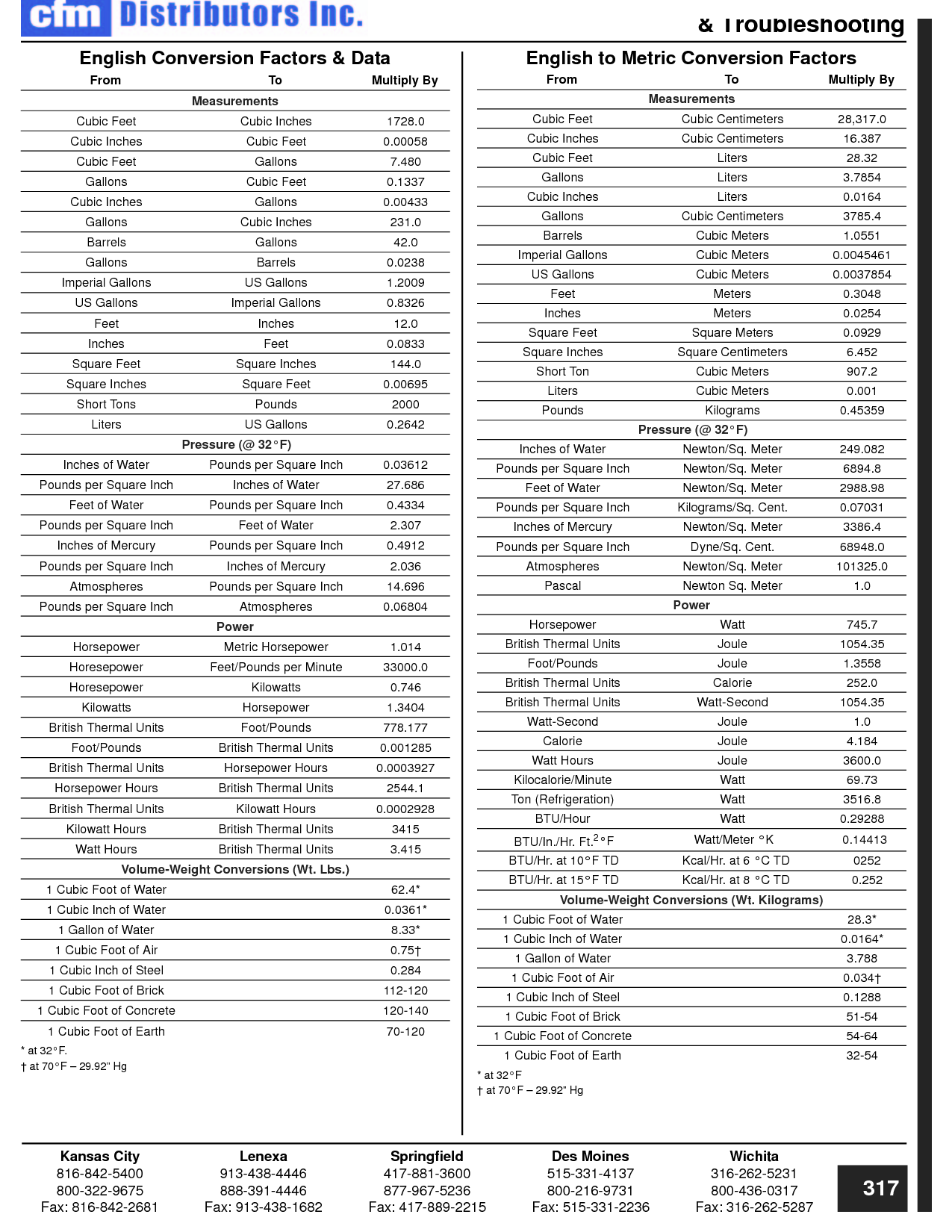
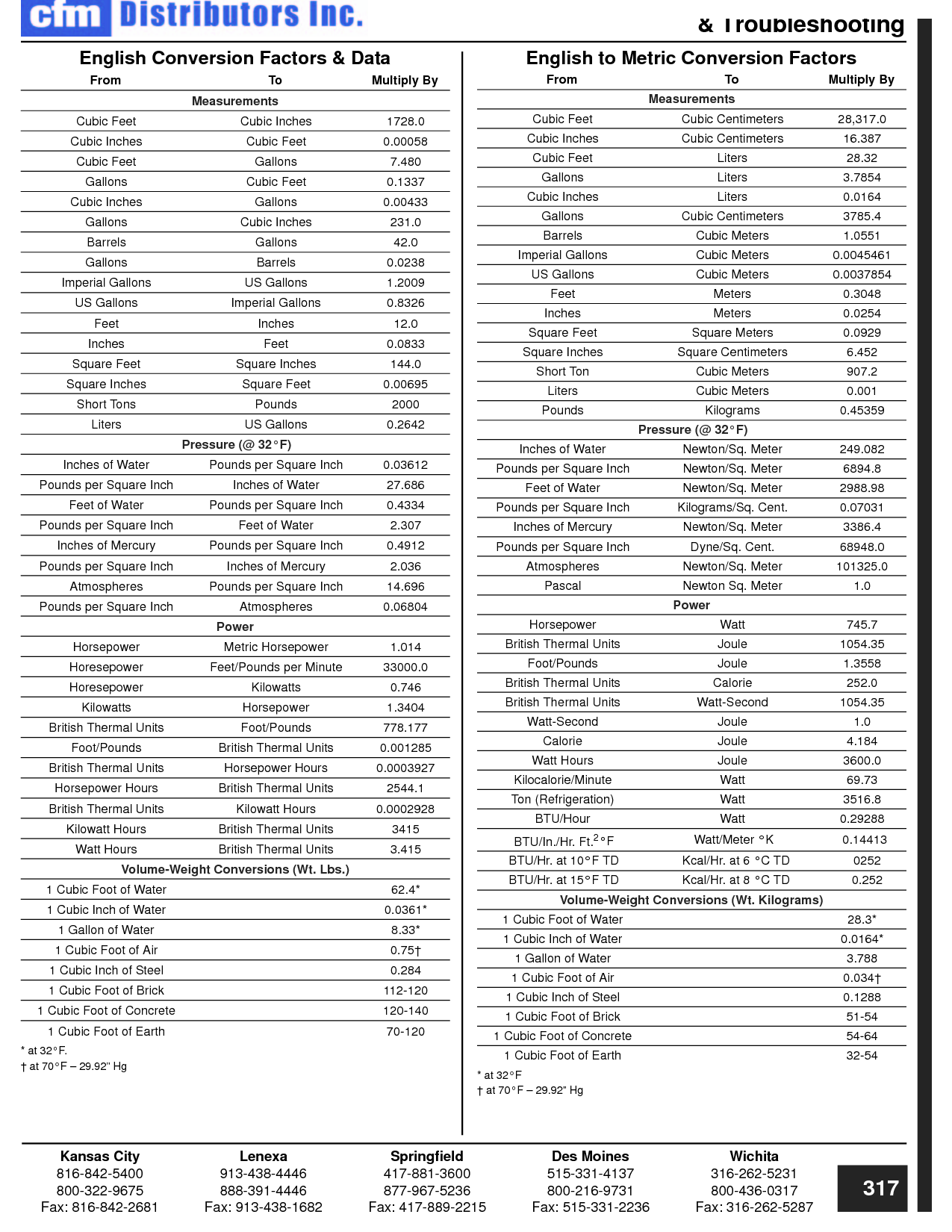
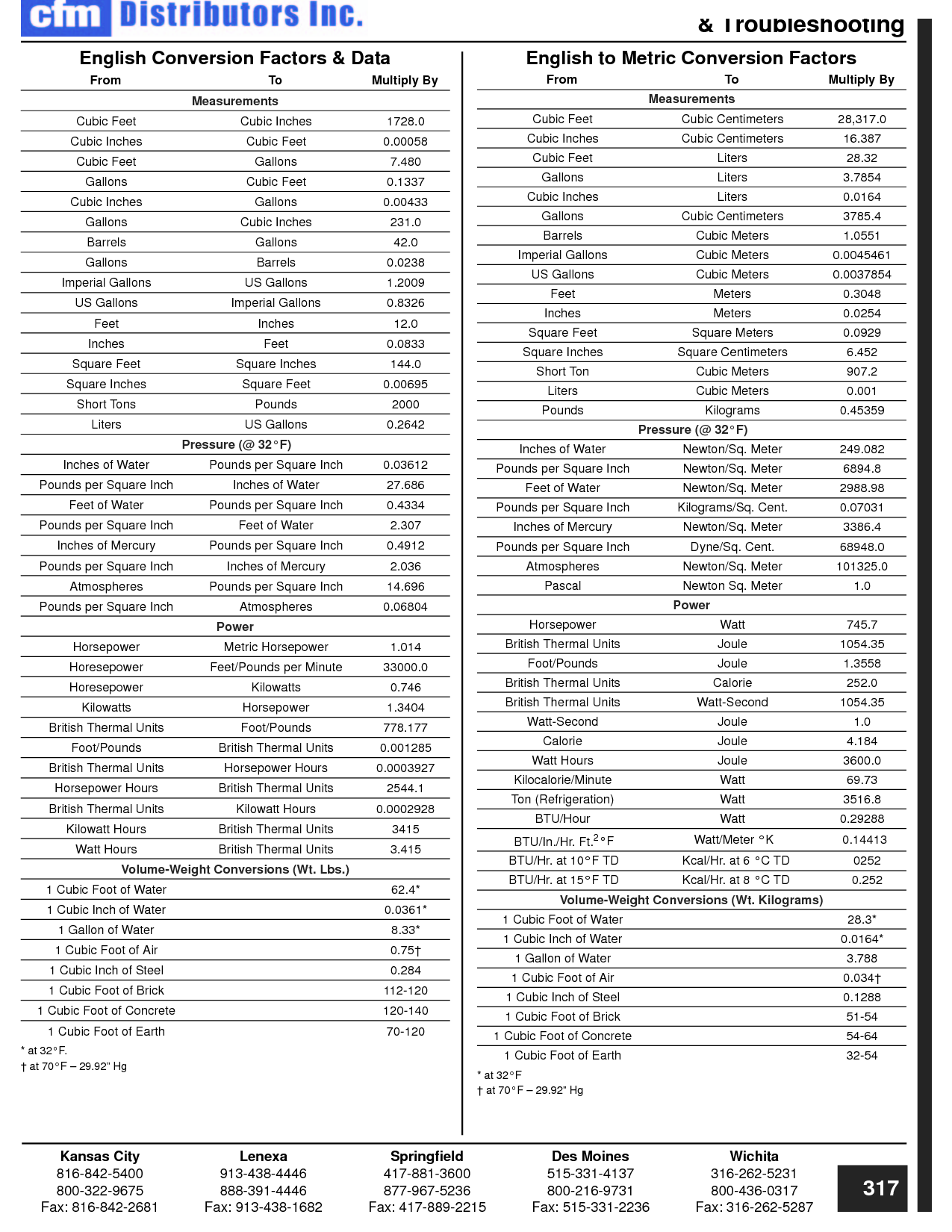
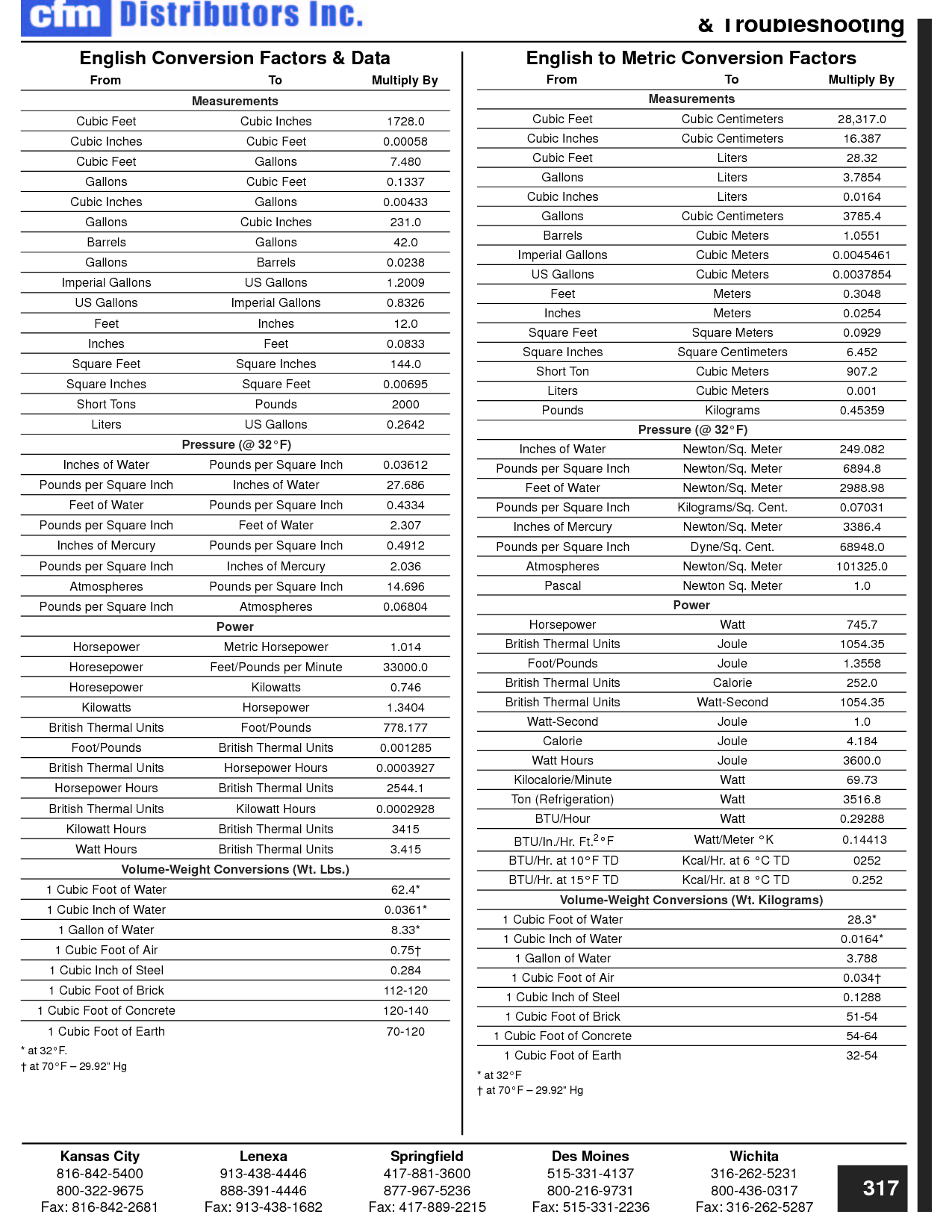
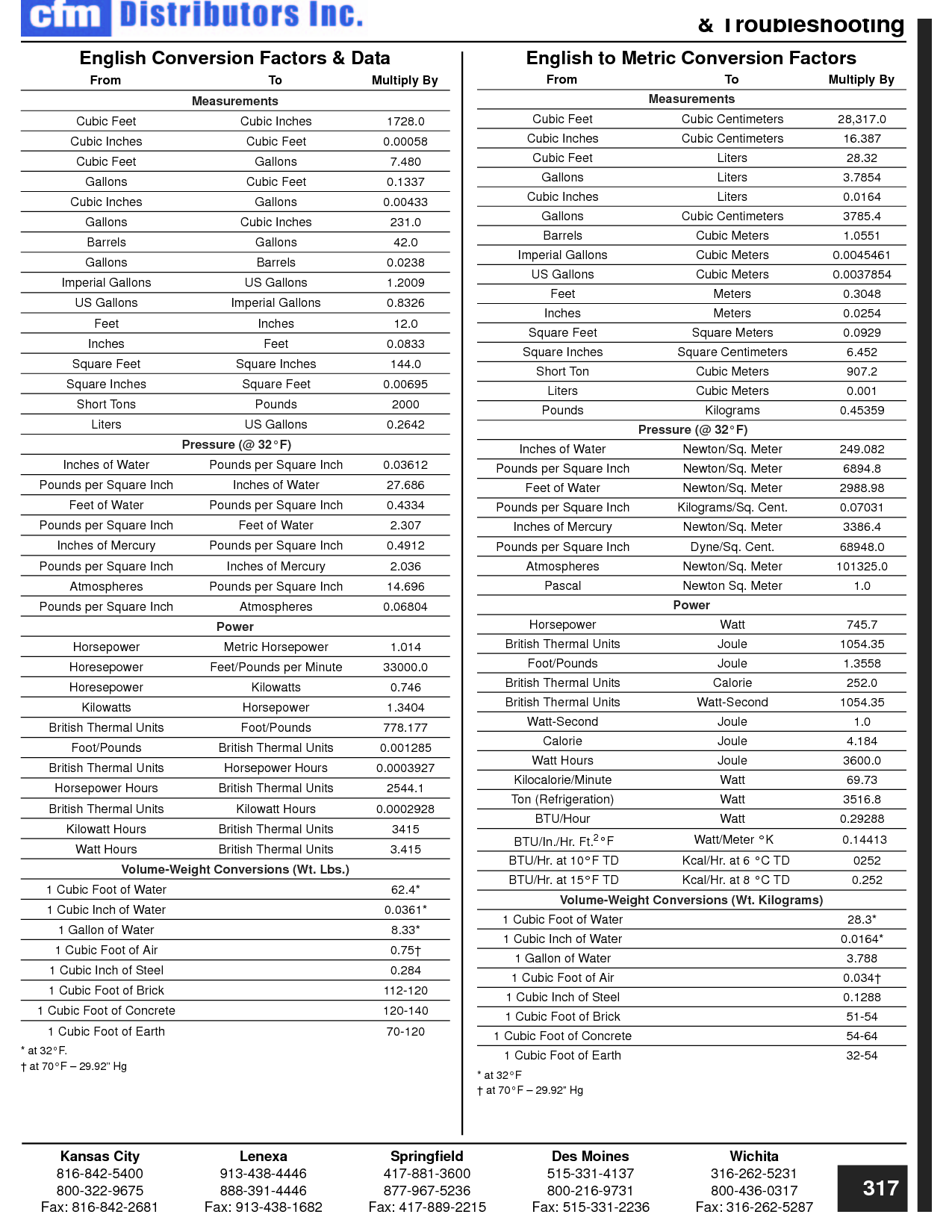
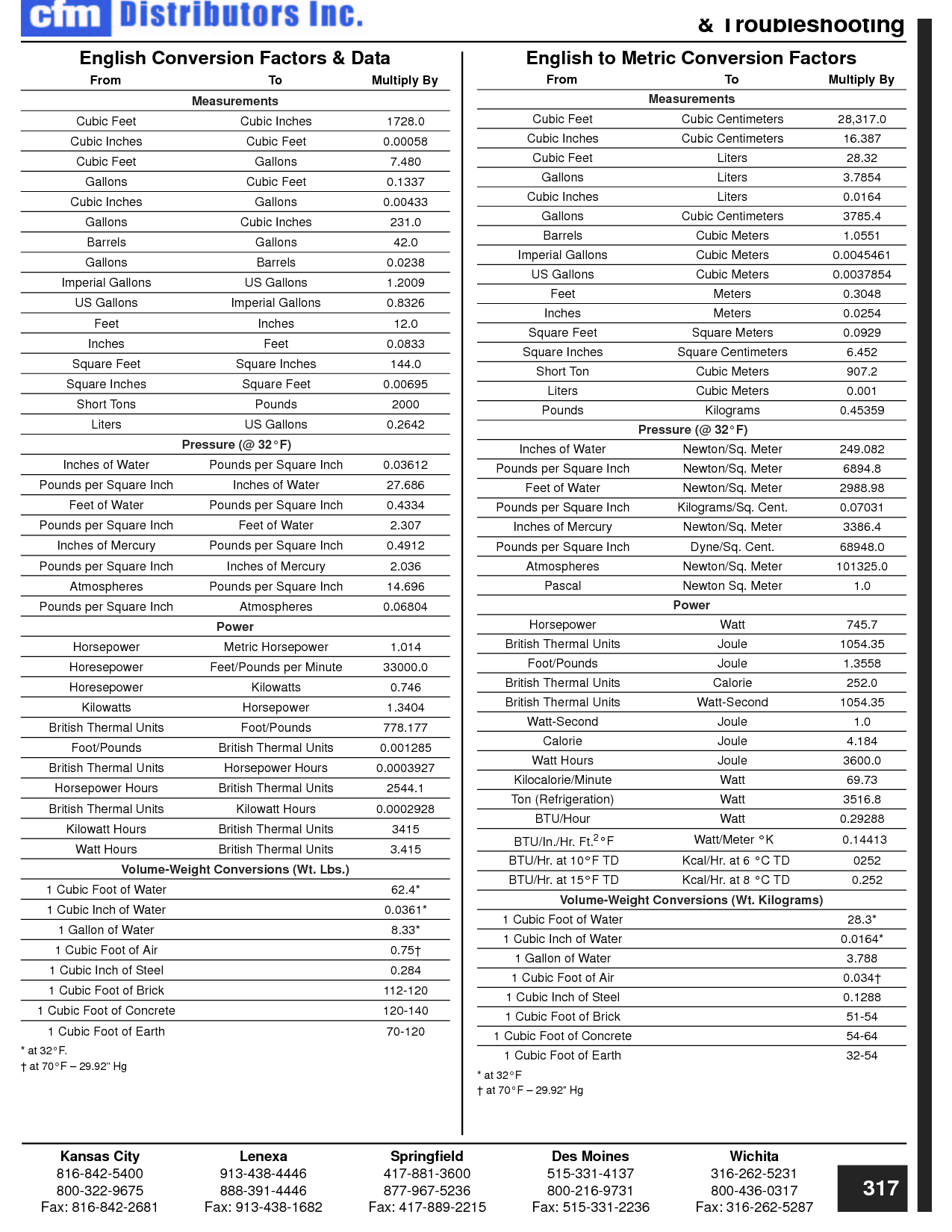
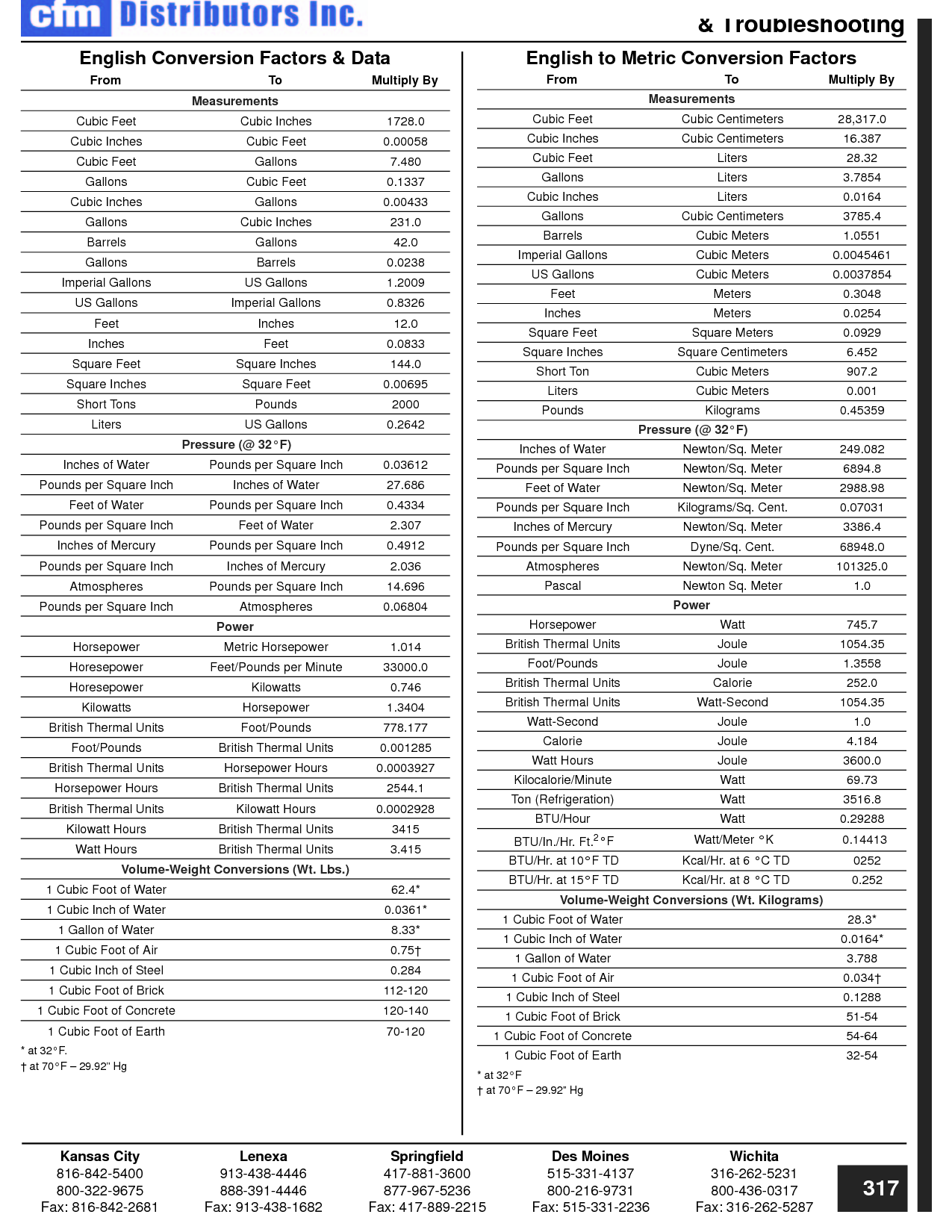
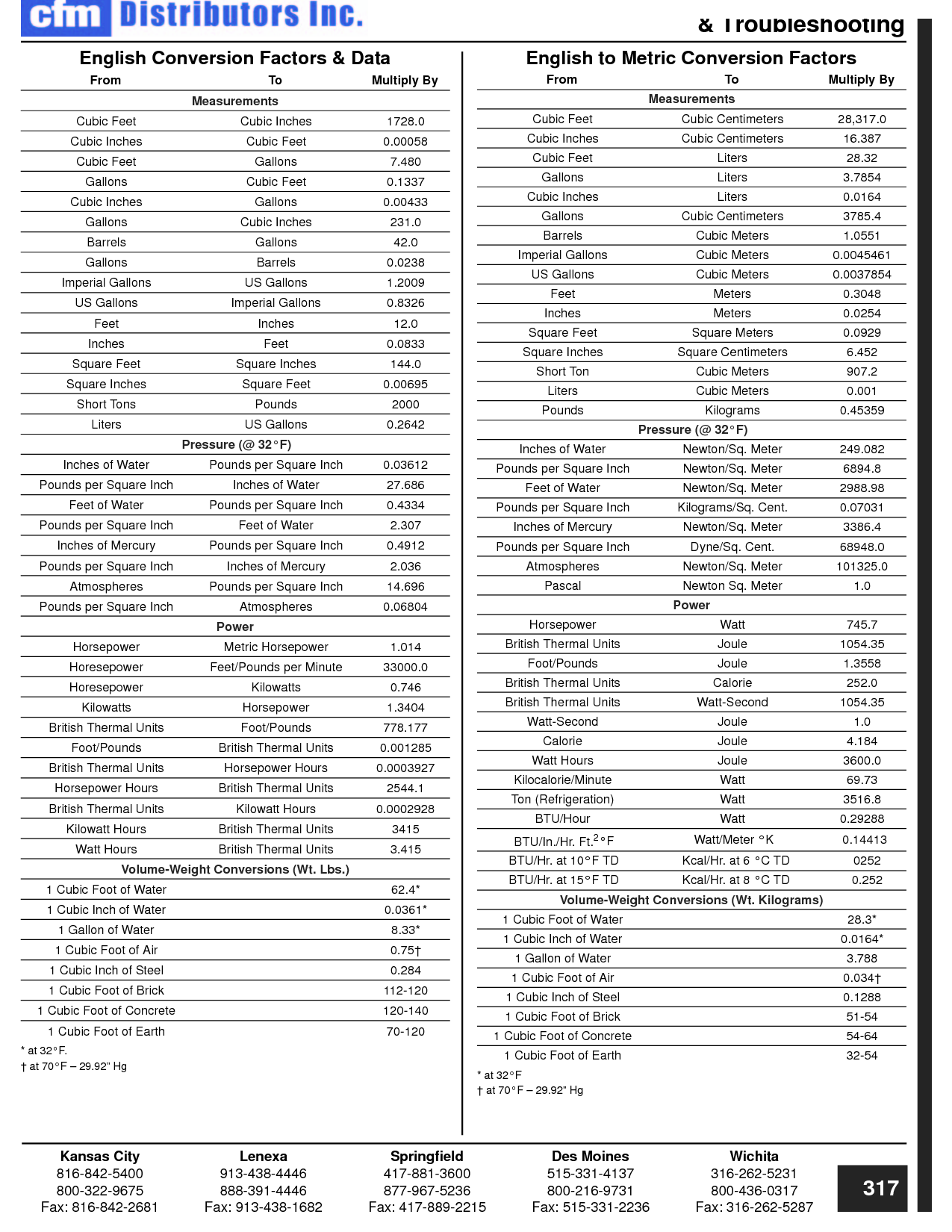
How can you convert between different units of measurement?
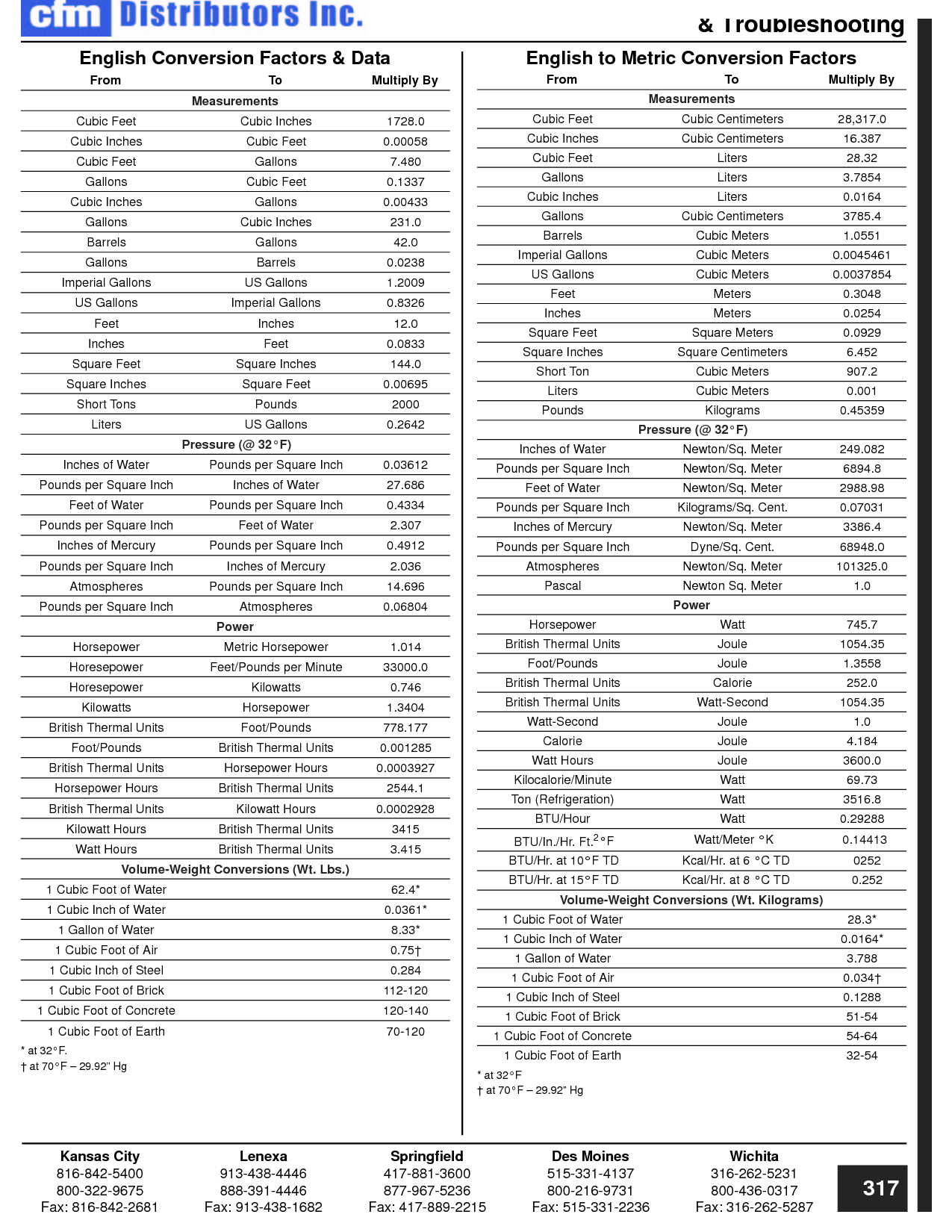
To convert between different units of measurement, you need to know the conversion factor between the two units. Just multiply the measurement you want to convert by the appropriate conversion factor to get the equivalent measurement in the new unit. Make sure to pay attention to the direction of the conversion (e.g., if you are converting from smaller to larger units, you would multiply; if converting from larger to smaller units, you would divide). Practice and familiarity with common conversion factors can help make the process easier and quicker.
What is the significance of significant figures in chemistry?
Significant figures are important in chemistry as they provide a way to communicate the precision of measurements. By using the correct number of significant figures, scientists can ensure the accuracy and reliability of their data and calculations. It also helps in understanding the uncertainties associated with measurements and ensures that the final result is reported with the appropriate level of accuracy.
What is the difference between a physical property and a chemical property?
Physical properties are characteristics of a substance that can be observed or measured without changing its chemical composition, such as color, density, and melting point. On the other hand, chemical properties describe how a substance interacts with other substances to form new compounds, such as flammability, reactivity, and toxicity. Essentially, physical properties are about the appearance and behavior of a substance, while chemical properties involve its ability to undergo chemical reactions and transform into new substances.
How is the density of a substance calculated?
The density of a substance is calculated by dividing the mass of the substance by its volume. The formula for calculating density is Density = mass/volume. Mass is usually measured in grams or kilograms, while volume is typically measured in cubic centimeters or cubic meters.
Why is it important to use proper units and labels when reporting measurements?
Using proper units and labels when reporting measurements is important because it provides clarity and precision to the information being communicated. Units help to define the quantity being measured and allow for easy comparability and understanding across different contexts. Labels provide context and help prevent misunderstandings or errors in interpreting the data. Without proper units and labels, measurements can be confusing or meaningless, leading to incorrect analysis, decision-making, or communication of findings.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.

















Comments