Ph Worksheet Key
Are you in search of a practical and effective learning tool to enhance your understanding of subject-verb agreement? Look no further! Worksheets provide an excellent way for students to practice identifying the subject and corresponding verb within a sentence, making it easier to grasp the concept and improve grammatical accuracy in their writing.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
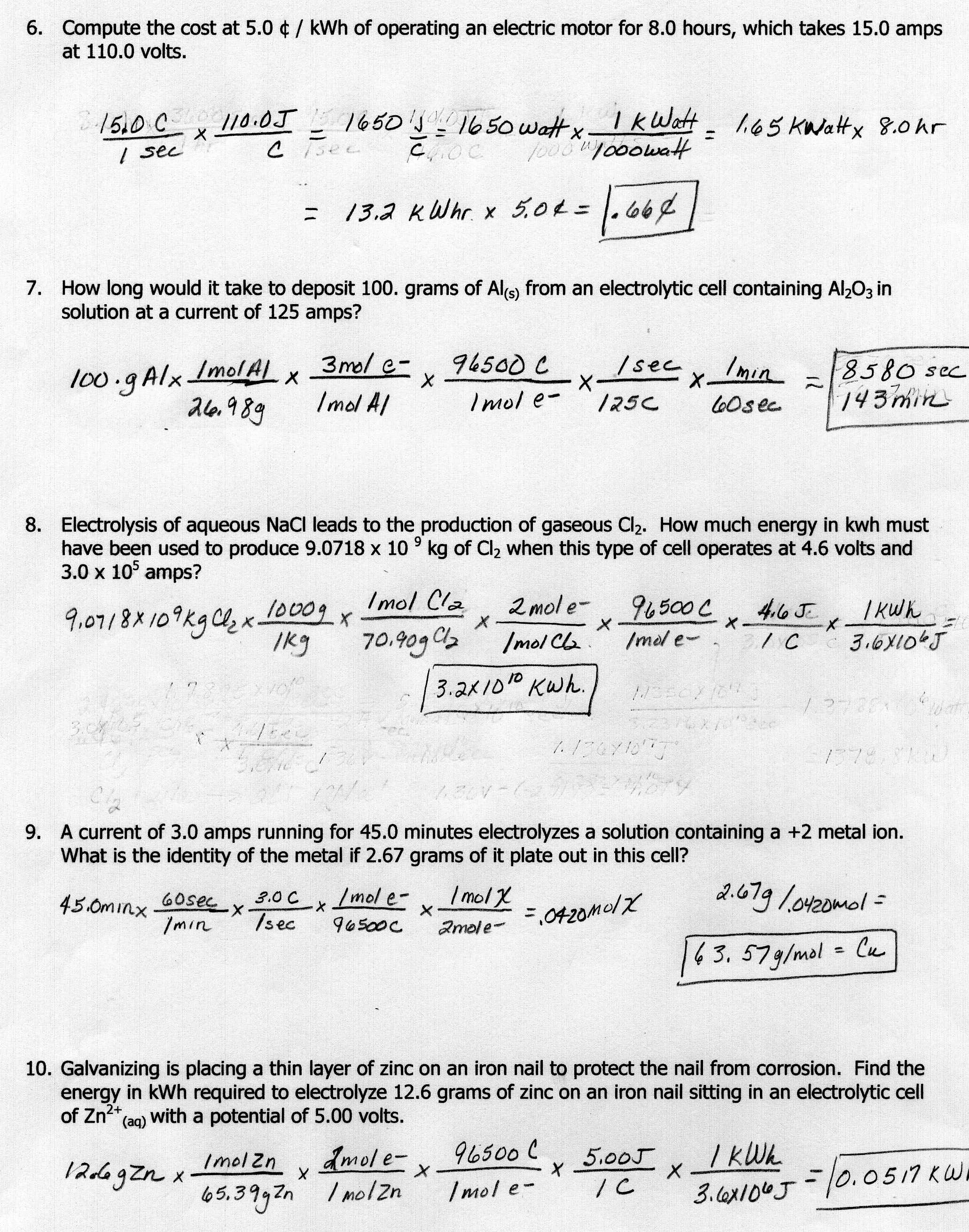
What is the formula for calculating pH?
The formula for calculating pH is pH = -log[H+], where [H+] represents the concentration of hydrogen ions in a solution.
What does pH measure?
pH measures the acidity or alkalinity of a solution on a scale from 0 to 14, with 7 being neutral. A pH below 7 indicates acidity, while a pH above 7 indicates alkalinity. pH is determined by the concentration of hydrogen ions in the solution, with lower pH values having higher concentrations of hydrogen ions and higher pH values having lower concentrations.
How is pH related to hydrogen ion concentration?
pH is a measure of the concentration of hydrogen ions in a solution. As the concentration of hydrogen ions increases, the pH of the solution decreases, and vice versa. This relationship is described by the formula pH = -log[H+], where [H+] represents the molar concentration of hydrogen ions. Therefore, a lower pH value indicates a higher concentration of hydrogen ions, and a higher pH value indicates a lower concentration of hydrogen ions in a solution.
What is the pH of a neutral solution?
The pH of a neutral solution is 7.
Define an acidic solution.
An acidic solution is a solution that has a pH level lower than 7, indicating a high concentration of hydrogen ions. This higher concentration of hydrogen ions makes the solution capable of donating protons in chemical reactions, leading to its acidic properties such as sour taste, ability to corrode metals, and turn blue litmus paper red.
Define a basic (alkaline) solution.
A basic (alkaline) solution is a solution with a pH greater than 7 on the pH scale, indicating a higher concentration of hydroxide ions (OH-) compared to hydronium ions (H+). Basic solutions typically have a bitter taste, feel slippery on the skin, and can turn litmus paper blue. Common examples of basic solutions include soap, baking soda, and bleach.
What is the pH value of pure water?
The pH value of pure water is 7, which is considered neutral on the pH scale. This means that pure water is neither acidic nor basic, as it contains equal concentrations of hydrogen ions (H+) and hydroxide ions (OH-).
How does pH affect the properties of substances?
pH plays a significant role in determining the properties of substances by influencing their chemical reactions, solubility, and interactions with other molecules. pH affects the charge of molecules, which in turn impacts their ability to bind with other molecules or change their physical state. For example, at extreme pH levels, proteins can denature and lose their function, while certain substances may become more soluble or reactive. Overall, pH levels can alter the behavior and properties of substances by changing their molecular structure and interactions.
How can pH be measured experimentally?
pH can be measured experimentally using a pH meter, pH paper or pH indicator solutions. pH meters are electronic devices that provide a digital readout of the pH level of a solution by measuring the voltage of a special pH-sensitive electrode. pH paper changes color based on the pH level of the solution it is dipped into, allowing for a rough estimate of the pH range. pH indicator solutions change color based on the pH level of the solution they are added to, providing a visual indication of the pH level.
Provide an example of a substance with a pH value of 2.
One example of a substance with a pH value of 2 is stomach acid, also known as gastric acid. Stomach acid is a digestive fluid composed primarily of hydrochloric acid, and it plays a crucial role in breaking down food in the stomach for digestion.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.






















Comments