Percent Composition Worksheet Answer Key
Are you a chemistry student looking for a reliable and comprehensive resource to help you understand percent composition? Look no further! In this blog post, we will provide you with a detailed answer key to a percent composition worksheet, allowing you to practice and master this fundamental concept in your studies. Whether you are preparing for an exam or simply want to deepen your understanding, this worksheet answer key will be a valuable tool for you.
Table of Images 👆
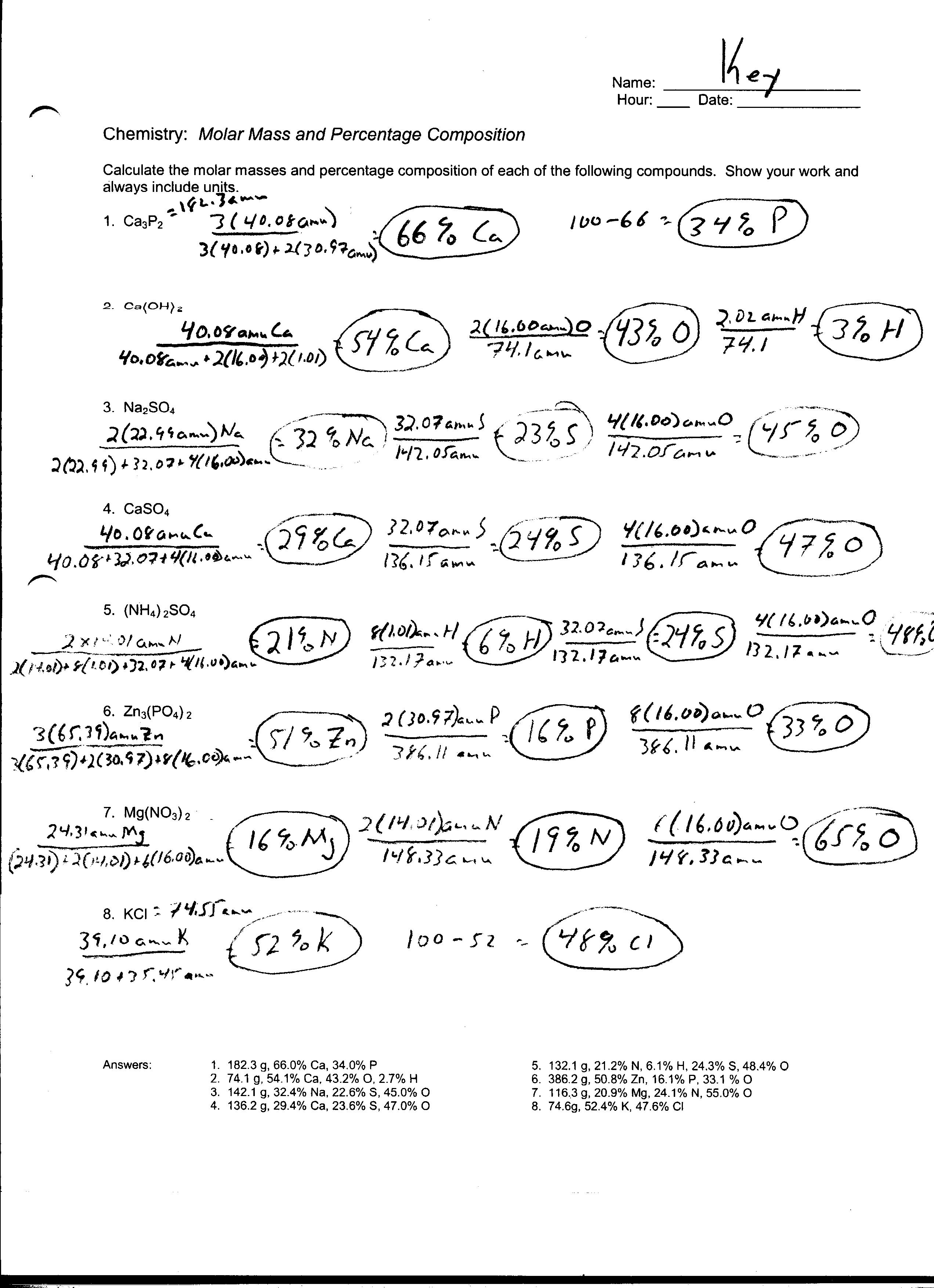
- Percent Composition Worksheet
- Chemistry Percent Composition Worksheet
- Percent Composition Empirical Formula Worksheet and Answers
- Empirical Formula Worksheet Answer Key
- Mass to Mole Stoichiometry Worksheet Answer Key
- Percent Composition Empirical Formula Worksheet Answers
- Chemistry Stoichiometry Worksheet Answer Key
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is percent composition?
Percent composition is a way to express the relative amount of each element in a compound. It is calculated by dividing the mass of an individual element by the total molar mass of the compound, and then multiplying by 100 to express it as a percentage. Percent composition is useful in determining the properties and behavior of a compound, as well as in analyzing and comparing different chemical substances.
How is percent composition calculated?
Percent composition is calculated by dividing the mass of a specific element in a compound by the total molar mass of the compound, then multiplying by 100. This value represents the percentage of the total mass of the compound that is due to that specific element. It can be used to determine the relative amounts of different elements in a compound.
Why is percent composition important in chemistry?
Percent composition is important in chemistry because it allows scientists to determine the quantity of elements present in a compound, which is crucial for understanding the chemical properties and behavior of substances. By calculating the percentage of each element in a compound, chemists can predict reactivity, molar masses, and stoichiometry of reactions. This knowledge is essential for designing and formulating new materials, determining the purity of substances, and ensuring the accuracy of experiments and reactions in chemical analysis and synthesis.
What information does percent composition provide about a compound?
Percent composition provides information about the relative proportion of each element present in a compound by expressing the mass of each element as a percentage of the total mass of the compound. This information helps in determining the chemical formula and structure of the compound, as well as in predicting its physical and chemical properties.
How can percent composition be used to determine the empirical formula of a compound?
Percent composition can be used to determine the empirical formula of a compound by calculating the mass percentage of each element present in the compound. Once the mass percentages are known, the number of moles of each element can be determined based on the assumption that the total mass of the compound is composed of these elements. The mole ratios of the elements are then calculated and simplified to find the simplest whole number ratio, which represents the empirical formula of the compound.
What is the difference between empirical formula and molecular formula?
The empirical formula represents the simplest whole number ratio of different atoms present in a compound, while the molecular formula provides the actual number of each type of atom in a molecule. The empirical formula helps determine the basic composition of a compound, while the molecular formula gives a more detailed picture of the actual arrangement of atoms in a molecule.
How does percent composition relate to stoichiometry in chemical reactions?
Percent composition refers to the percentage by mass of each element in a compound. In stoichiometry, this information is crucial because it allows for the determination of the relative quantities of reactants and products in a chemical reaction. By knowing the percent composition of a compound, one can calculate the molar ratios of the elements involved in the reaction, ensuring that the reaction proceeds according to the correct stoichiometry. This information is essential for predicting the amounts of reactants needed, products formed, and for balancing chemical equations accurately.
How can percent composition be used to determine the mass of a specific element in a compound?
Percent composition can be used to determine the mass of a specific element in a compound by first converting the percentage of that element in the compound to a decimal, then multiplying it by the total mass of the compound. This calculation allows you to find the mass of the specific element present in the compound. For example, if a compound has a percent composition of 40% oxygen and a total mass of 100 grams, you would convert 40% to 0.40, then multiply 0.40 by 100 grams to find that there are 40 grams of oxygen in the compound.
How is percent composition used in determining the purity of a sample?
Percent composition is used in determining the purity of a sample by comparing the actual percentage of a particular element in the sample to the theoretical percentage if the sample were pure. By calculating the percent composition of the sample and comparing it to the expected value for a pure substance, one can assess the extent of impurities present in the sample and determine its overall purity. This method is especially useful in analytical chemistry to quantify the quality of a sample and ensure it meets desired standards.
How does the percent composition of a compound affect its physical and chemical properties?
The percent composition of a compound plays a significant role in determining its physical and chemical properties. The types and quantities of elements present in a compound influence factors such as melting point, boiling point, density, color, solubility, and reactivity. For example, a higher concentration of a particular element may lead to stronger intermolecular forces, affecting the compound's boiling or melting point. Additionally, the specific elements present can determine the compound's ability to participate in chemical reactions, influencing its reactivity. Overall, the percent composition of a compound is a key factor in defining its unique physical and chemical characteristics.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.

























Comments