Electron Dot Model Worksheets
Electron Dot Model Worksheets are an invaluable resource for students studying chemistry or anyone who wants to learn more about electron structures and chemical bonding. These worksheets provide a structured and organized way to practice and master the concepts of electron dot models, making them perfect for high school and college-level students seeking to improve their understanding of this fundamental topic.
Table of Images 👆
More Dot Worksheets
Dot to Dot WorksheetsHard Dot to Dot Worksheets
Printable Dot to Dot Worksheets 1-100
Butterfly Dot to Dot Free Printable Preschool Worksheet
Jesus Connect the Dots Worksheets
Constellation Connect the Dots Worksheet
Halloween Connect the Dots Worksheets
Coordinate Plane Connect Dots Worksheets
Dr. Seuss Connect the Dots Worksheets
Christian Connect the Dots Worksheets
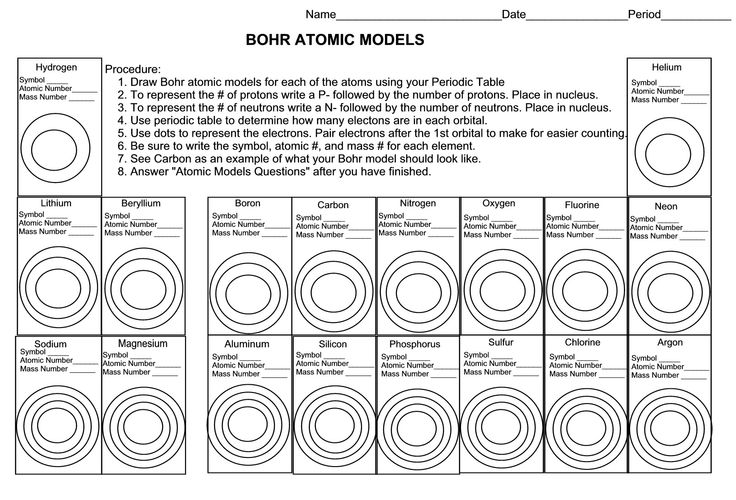
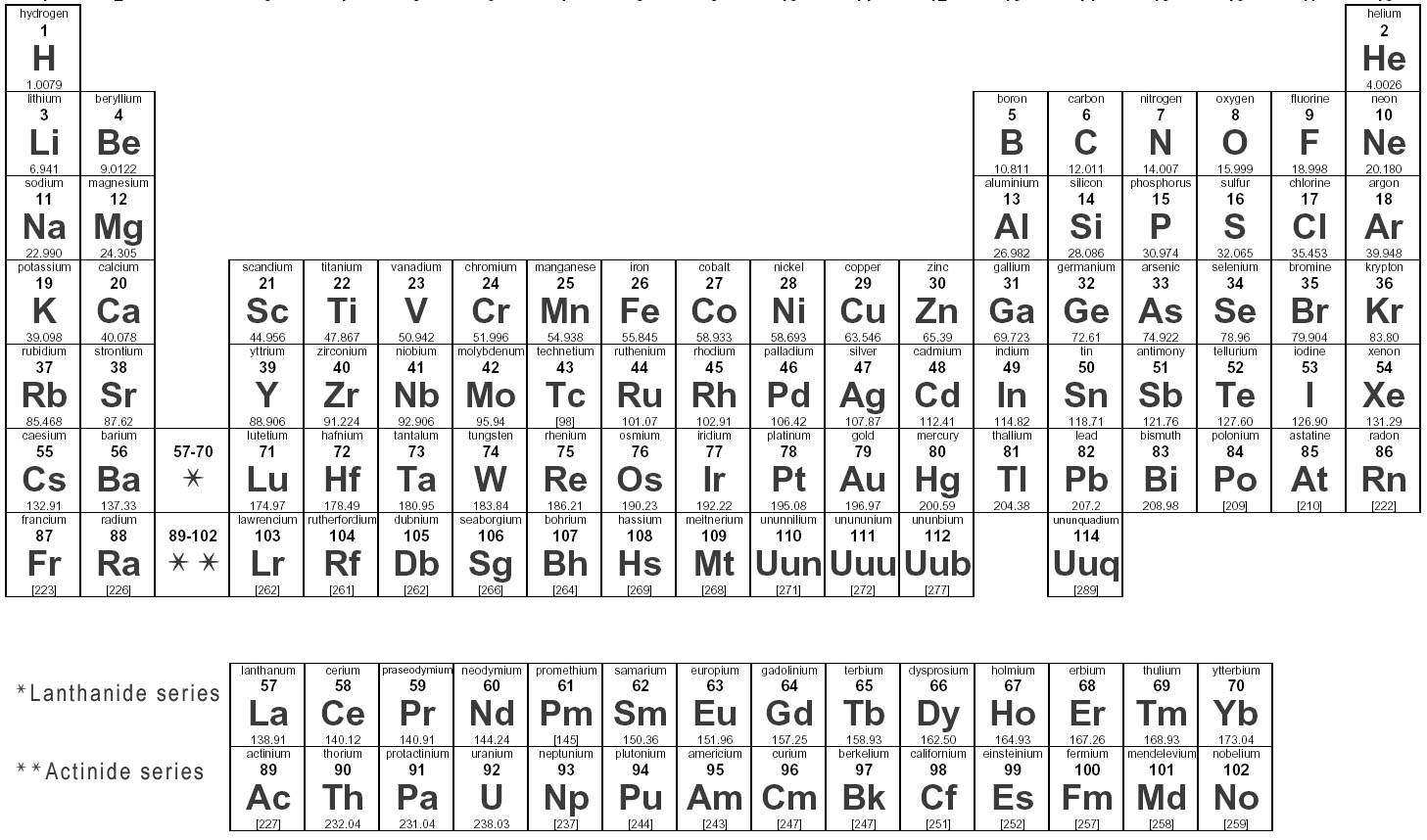
What is the Electron Dot Model?
The Electron Dot Model, also known as Lewis Dot Structure or Lewis Electron Dot Diagram, is a symbolic representation of the valence electrons in an atom. In this model, the valence electrons are shown as dots around the atomic symbol, with each dot representing a single electron. The arrangement of these dots illustrates the bonding between atoms in a molecule and helps in predicting the chemical properties of the elements involved in the bonding.
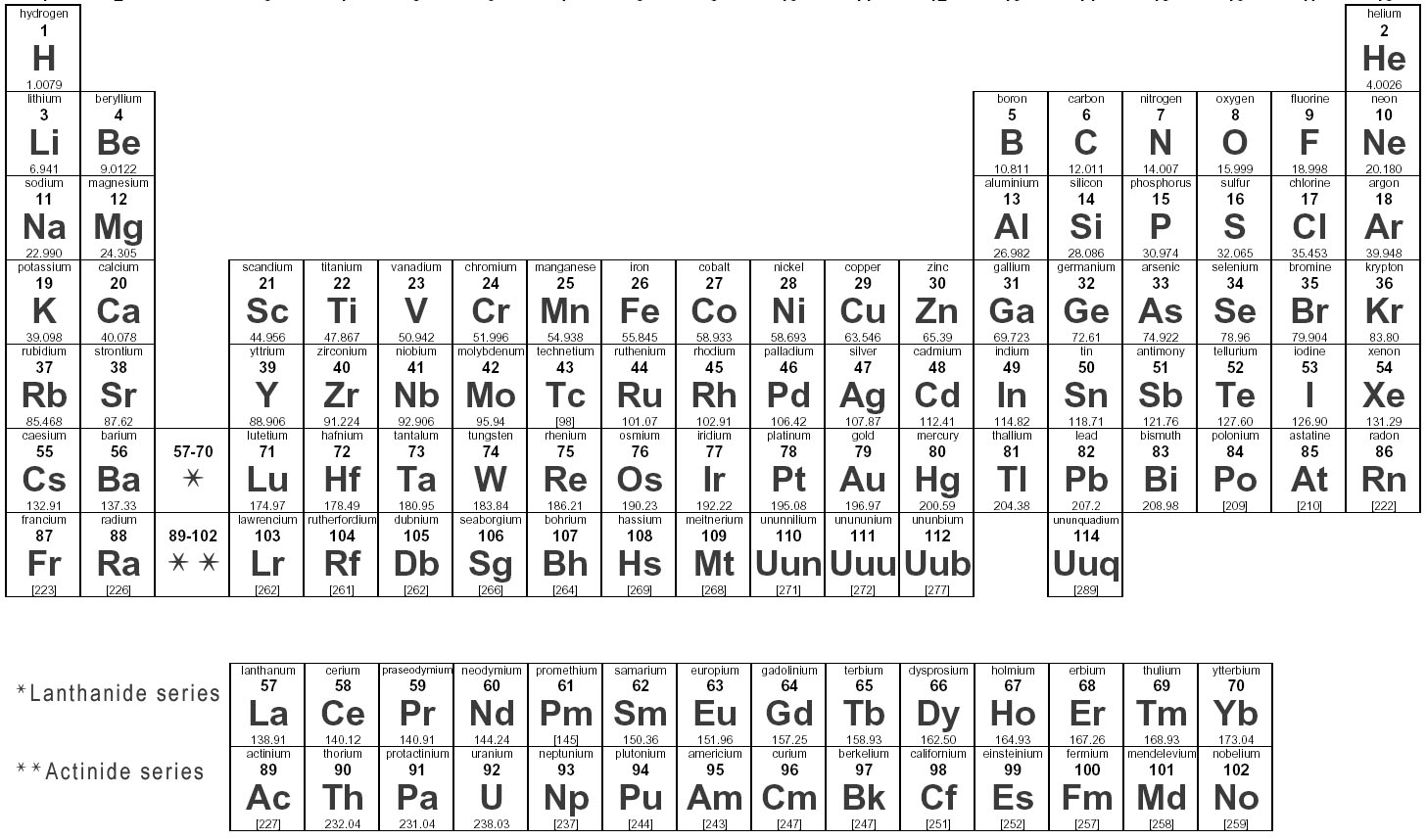
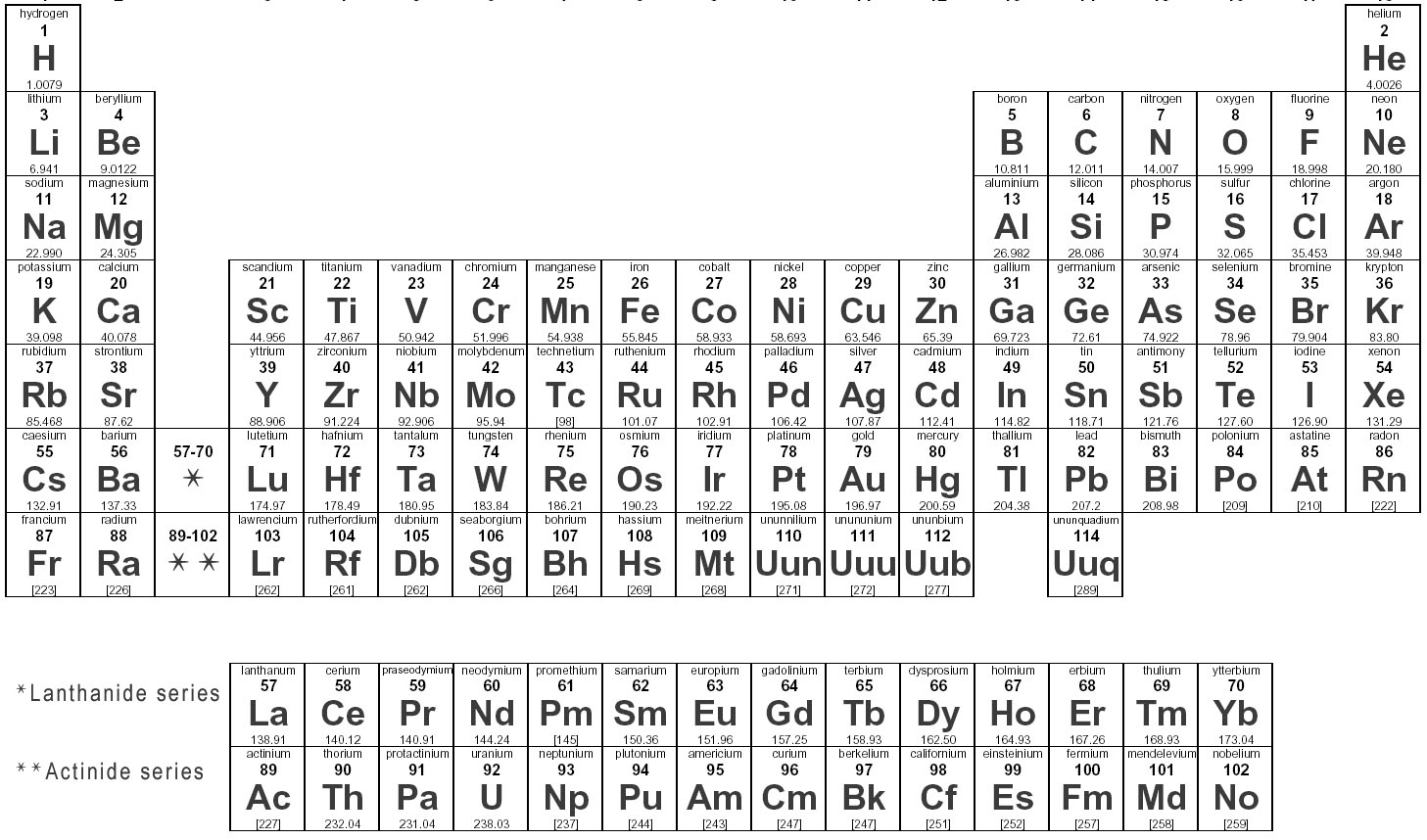
How many valence electrons does an element in Group 1 have?
An element in Group 1 has 1 valence electron.
How many valence electrons does an element in Group 17 have?
An element in Group 17 has 7 valence electrons.
Write the electron dot symbol for oxygen.
The electron dot symbol for oxygen contains 6 dots, with 4 dots placed on two sides of the O symbol indicating the 6 valence electrons of oxygen, with two dots on each side paired up.
Which element has the electron dot symbol: X:?
The element with the electron dot symbol X: is Xenon.
What is the maximum number of valence electrons an atom can have?
The maximum number of valence electrons an atom can have is 8. This is known as the octet rule, where atoms tend to gain, lose, or share electrons to achieve a stable electron configuration similar to the noble gases with 8 valence electrons.
How do you determine if an atom will gain or lose electrons to form ions?
Atoms will gain or lose electrons to form ions based on their valence electron configuration. Atoms tend to gain or lose electrons to achieve a more stable electron configuration, typically following the octet rule. If an atom has fewer than four valence electrons, it will tend to lose electrons to attain a full outer shell. On the other hand, if an atom has more than four valence electrons, it will tend to gain electrons to complete its outer shell. This process allows atoms to achieve a more stable electron configuration, thus forming positively or negatively charged ions.
Why do atoms form bonds?
Atoms form bonds to achieve stability by filling their outermost electron shells. This allows them to reach a more energetically favorable state. By sharing, losing, or gaining electrons through bonding, atoms can achieve a full outer shell of electrons, following the octet rule, which is a stable configuration similar to that of noble gases. Bond formation allows atoms to decrease their energy levels and become more chemically stable.
What is the difference between ionic and covalent bonding?
Ionic bonding occurs when one atom donates electrons to another, resulting in the formation of positively and negatively charged ions that attract each other. Covalent bonding, on the other hand, involves atoms sharing electrons to achieve a more stable electron configuration. In ionic bonding, electrons are transferred, while in covalent bonding, electrons are shared between atoms.
What is the Lewis structure for water?
The Lewis structure for water (H2O) consists of two hydrogen atoms bonded to an oxygen atom, with two pairs of nonbonding electrons (lone pairs) on the oxygen atom. This results in a bent molecular geometry with a bond angle of approximately 104.5 degrees.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



















Comments