Mole Worksheets with Answer Keys
Are you searching for mole worksheets that come with answer keys? Look no further! We understand the importance of having worksheets that provide both a comprehensive set of questions and the corresponding answers. Whether you are a student studying chemistry or a teacher looking to supplement your lessons, our mole worksheets with answer keys are designed to help you grasp this fundamental concept with ease and accuracy.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is a mole in chemistry?
A mole is a unit of measurement in chemistry that represents the amount of substance containing the same number of entities (atoms, molecules, ions, etc.) as there are in 12 grams of carbon-12. It is a fundamental concept used to quantify amounts of substances in chemical reactions and is equal to approximately 6.022 x 10^23 entities, which is known as Avogadro's number.
How is the mole defined?
A mole is defined as the amount of a substance that contains as many elementary entities (such as atoms, molecules, ions, or electrons) as there are atoms in 12 grams of carbon-12. This number is approximately 6.022 x 10^23, known as Avogadro's number. The mole is a fundamental unit in chemistry and is used to express amounts of substances in a convenient way for calculations.
What is Avogadro's number?
Avogadro's number is a fundamental constant in chemistry and physics, representing the number of constituent particles (usually atoms or molecules) in one mole of a substance. It is approximately equal to 6.022 x 10^23 and is named after the Italian scientist Amedeo Avogadro.
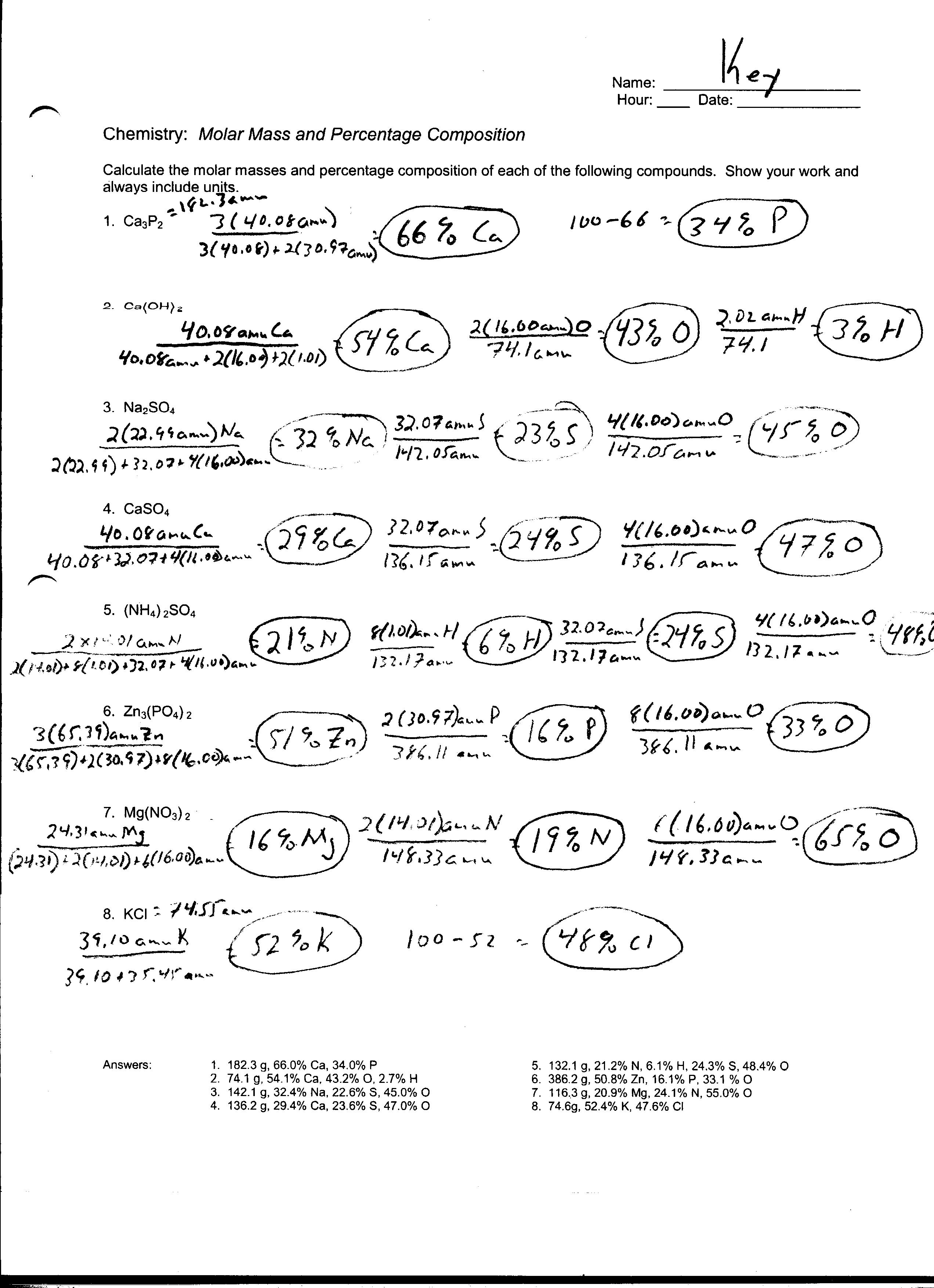
How is the molar mass of a compound calculated?
To calculate the molar mass of a compound, you first need to determine the molar mass of each element within the compound by looking up their atomic masses on the periodic table. Then, you multiply the atomic mass of each element by the number of atoms of that element in the compound, and finally add up the products for all elements present in the compound. This sum gives you the molar mass of the compound, which is expressed in grams per mole.
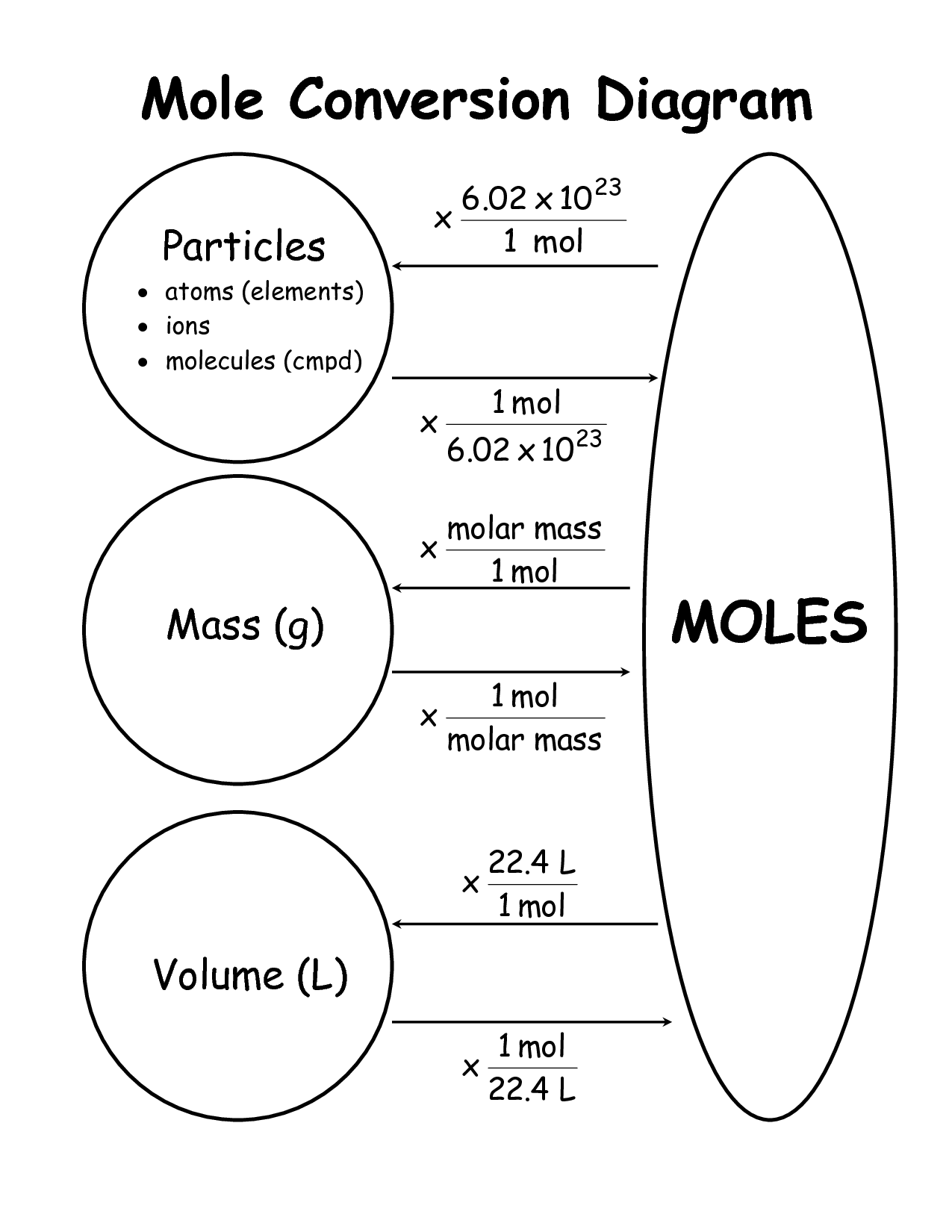
What is the relationship between a mole and a gram of a substance?
The relationship between a mole and a gram of a substance is defined by the substance's molar mass. One mole of any substance contains Avogadro's number of particles, which is approximately 6.022 x 10^23 particles. The molar mass of a substance is the mass in grams of one mole of that substance. Therefore, the number of grams in a mole of a substance is equal to its molar mass.
How is stoichiometry related to moles?
Stoichiometry is directly related to moles as it involves the quantitative relationships between reactants and products in a chemical reaction. The coefficients in a balanced chemical equation represent the number of moles of each substance involved in the reaction, making moles a crucial unit of measurement in stoichiometry calculations. By using the mole ratio between reactants and products, stoichiometry allows us to determine the amounts of substances needed for a reaction and the amounts of products produced.
How is the mole used in chemical reactions?
The mole is used in chemical reactions to quantify the amount of a substance being used or produced. By converting the different units of mass, volume, or particles into moles, chemists can accurately calculate the quantities involved in a reaction. This allows for precise measurement, stoichiometric calculations, and the determination of reaction yields, making the mole an essential concept in chemistry.
What is the purpose of mole ratios in balancing equations?
The purpose of mole ratios in balancing equations is to ensure that the law of conservation of mass is obeyed. Mole ratios help in determining the amount of reactants needed to produce a certain amount of product, and vice versa. By balancing equations using mole ratios, we are able to accurately predict the quantities of substances involved in a chemical reaction, making it easier to calculate reaction yields and optimize the reaction conditions.
How can the mole concept be applied to determine the limiting reactant?
The mole concept can be used to determine the limiting reactant by comparing the amount of each reactant in terms of moles to the stoichiometry of the balanced chemical equation. By calculating the moles of each reactant involved in the reaction and comparing them to the stoichiometric ratios, we can identify which reactant is present in limited quantity and therefore limits the amount of product that can be formed. Whichever reactant produces the least amount of product based on the stoichiometry is the limiting reactant.
How are percent composition and empirical formula calculations related to moles?
Percent composition and empirical formula calculations are related to moles because they involve determining the proportion of different elements or compounds present in a substance based on the number of moles of each element. By calculating the moles of each element in a compound, one can determine the percent composition of each element within the compound. Additionally, knowing the moles of each element allows one to determine the simplest whole-number ratio of elements in a compound, which is essential for determining the empirical formula. Thus, moles play a crucial role in both percent composition and empirical formula calculations.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



















Comments