Writing Equations for Chemical Reactions Worksheet
Chemistry enthusiasts and students alike can greatly benefit from a thorough understanding of equations for chemical reactions. This worksheet is designed to assist learners in developing their equation-writing skills, focusing particularly on identifying and correctly representing entities and subjects within the reactions.
Table of Images 👆
- Balancing Chemical Equations Worksheet Answers
- Chemical Word Equations Worksheet Answers
- Types Chemical Reactions Worksheets Answers
- Balancing Chemical Equations Worksheet
- Balancing Chemical Equations Worksheet Answer Key
- Nuclear Reactions Worksheet
- Chemical Reaction Types Worksheet
- Life of Chemistry Vocabulary Practice Answers
- Sample of Chem 1A Lab Notebook
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
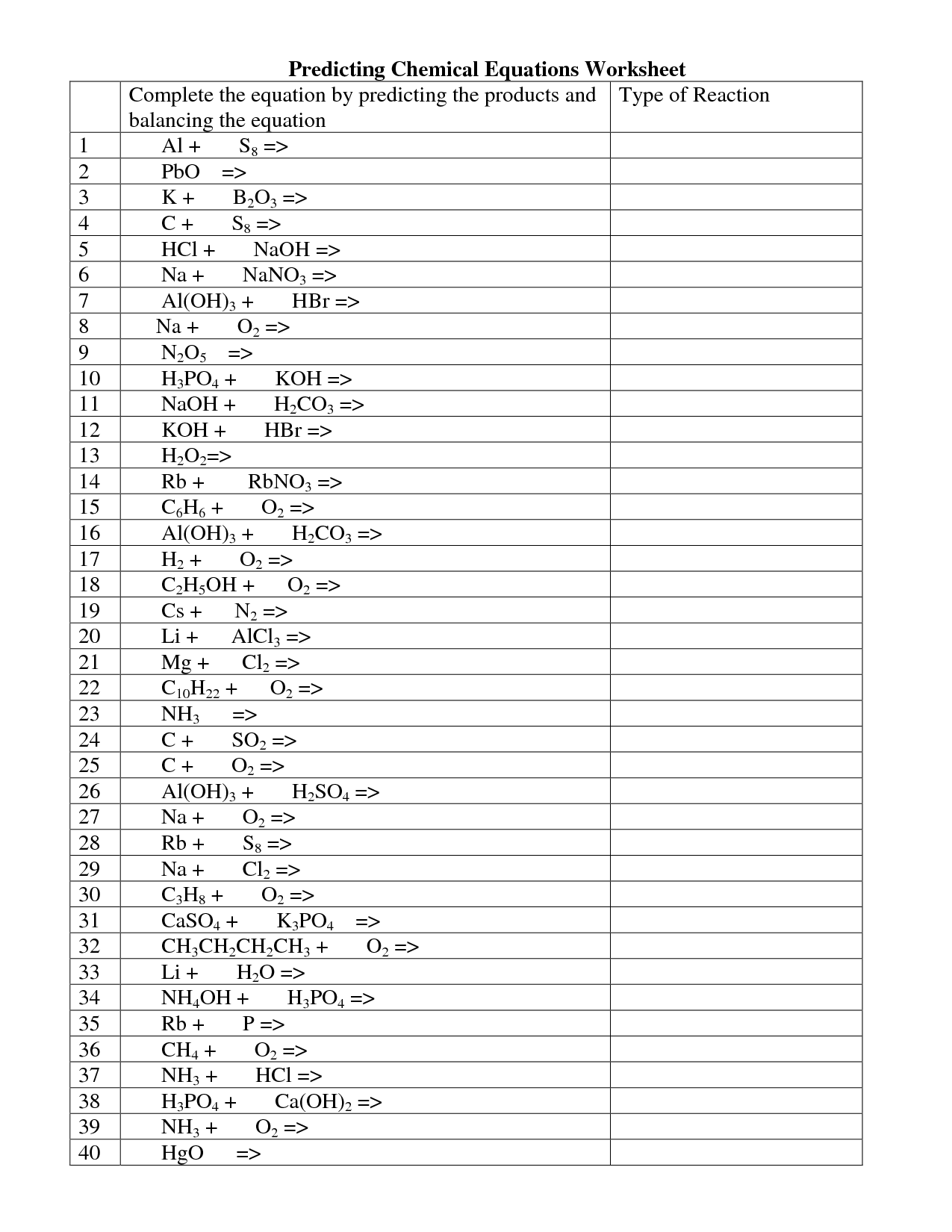
What is the first step in writing a chemical equation?
The first step in writing a chemical equation is to identify the reactants and products involved in the chemical reaction. This involves understanding the substances that are being combined or broken down in the reaction and determining the correct formulas for each chemical species.
How are reactants and products represented in a chemical equation?
Reactants are typically placed on the left side of a chemical equation and are the substances that are initially present and undergo a chemical reaction, while products are placed on the right side and are the substances that are formed as a result of the reaction. The reactants are separated from the products by an arrow symbol, indicating the direction of the reaction. Each reactant and product is represented by its chemical formula.
What do coefficients represent in a chemical equation?
Coefficients in a chemical equation represent the relative amounts of reactants and products involved in a reaction. They are used to balance the equation so that the number of atoms of each element on the reactant side is equal to the number of atoms of the same elements on the product side, ensuring that the law of conservation of mass is obeyed.
How do you balance a chemical equation?
To balance a chemical equation, you need to adjust the coefficients in front of the chemical formulas so that the number of atoms of each element is the same on both sides of the equation. Start by balancing the most complex compound or the one with the most atoms of different elements, then adjust the other compounds accordingly. Remember to only change the coefficients, not the subscripts, as this would change the identities of the compounds. Keep adjusting until the number of atoms of each element is equal on both sides, indicating a balanced equation.
What is the purpose of balancing a chemical equation?
Balancing a chemical equation ensures that the law of conservation of mass is obeyed, meaning that the number of atoms of each element on the reactant side must be equal to the number of atoms of that element on the product side. This is important because it shows the stoichiometry of the reaction, indicating the exact amount of each reactant and product involved in the chemical reaction.
What information can be determined from a balanced chemical equation?
A balanced chemical equation provides information on the types and quantities of reactants and products involved in a chemical reaction. It shows the ratio in which different substances combine or are produced in the reaction, allowing us to understand the stoichiometry of the reaction and predict the amounts of products that will be formed from a given amount of reactants. Additionally, it helps in determining the conservation of mass, as the total mass of the reactants must equal the total mass of the products in a balanced equation.
How do you determine the physical state of each reactant and product?
You can determine the physical state of each reactant and product by referring to the information provided in a chemical equation or reaction. Subscripts denoting the physical state are often included in parentheses following the chemical formula, such as (s) for solid, (l) for liquid, (g) for gas, and (aq) for aqueous (dissolved in water). By examining these symbols in the equation, you can identify the physical state of each substance involved in the reaction.
What are the different types of chemical reactions?
The different types of chemical reactions include synthesis (combination), decomposition, single displacement, double displacement, combustion, acid-base, and redox reactions. These reactions involve different rearrangements of atoms and bonds to form new substances with distinct chemical properties.
How can you identify the type of reaction from a given equation?
To identify the type of reaction from a given equation, you should first determine the reactants and products involved in the reaction. Then, analyze the changes in the chemical composition and bonding arrangements of the substances. Common types of reactions include synthesis (combination), decomposition, single replacement, double replacement, and combustion. By examining the reaction components and their rearrangement, you can classify the reaction based on the type of chemical transformation that has occurred.
What are the key rules and conventions for writing chemical equations?
When writing chemical equations, include the correct chemical formulas for reactants and products, balance the equation by adjusting coefficients, use the appropriate symbols (such as '+' for reactants and '?' for yields), and ensure the conservation of mass and charge on both sides of the equation. Additionally, follow the convention of writing states of matter (s for solid, l for liquid, g for gas, and aq for aqueous solutions) and use parentheses when necessary to indicate multiple molecules or compounds. Remember to include conditions for the reaction like temperature, pressure, or catalysts if relevant.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.































Comments