Writing Binary Compounds Worksheet
Are you having trouble understanding binary compounds and the rules for writing their formulas? Look no further! This blog post is designed to help students and chemistry enthusiasts master the art of writing binary compounds. Whether you are struggling with identifying the correct elements or simply need a refresher, this worksheet will provide you with a step-by-step approach to writing accurate and balanced formulas.
Table of Images 👆
- Naming Acids Worksheet Answer Key
- Chemical Formula Writing Worksheet
- Naming Covalent Compounds Worksheet
- Formulas with Polyatomic Ions Worksheet Answers
- Naming Ionic Compounds Worksheet Answers
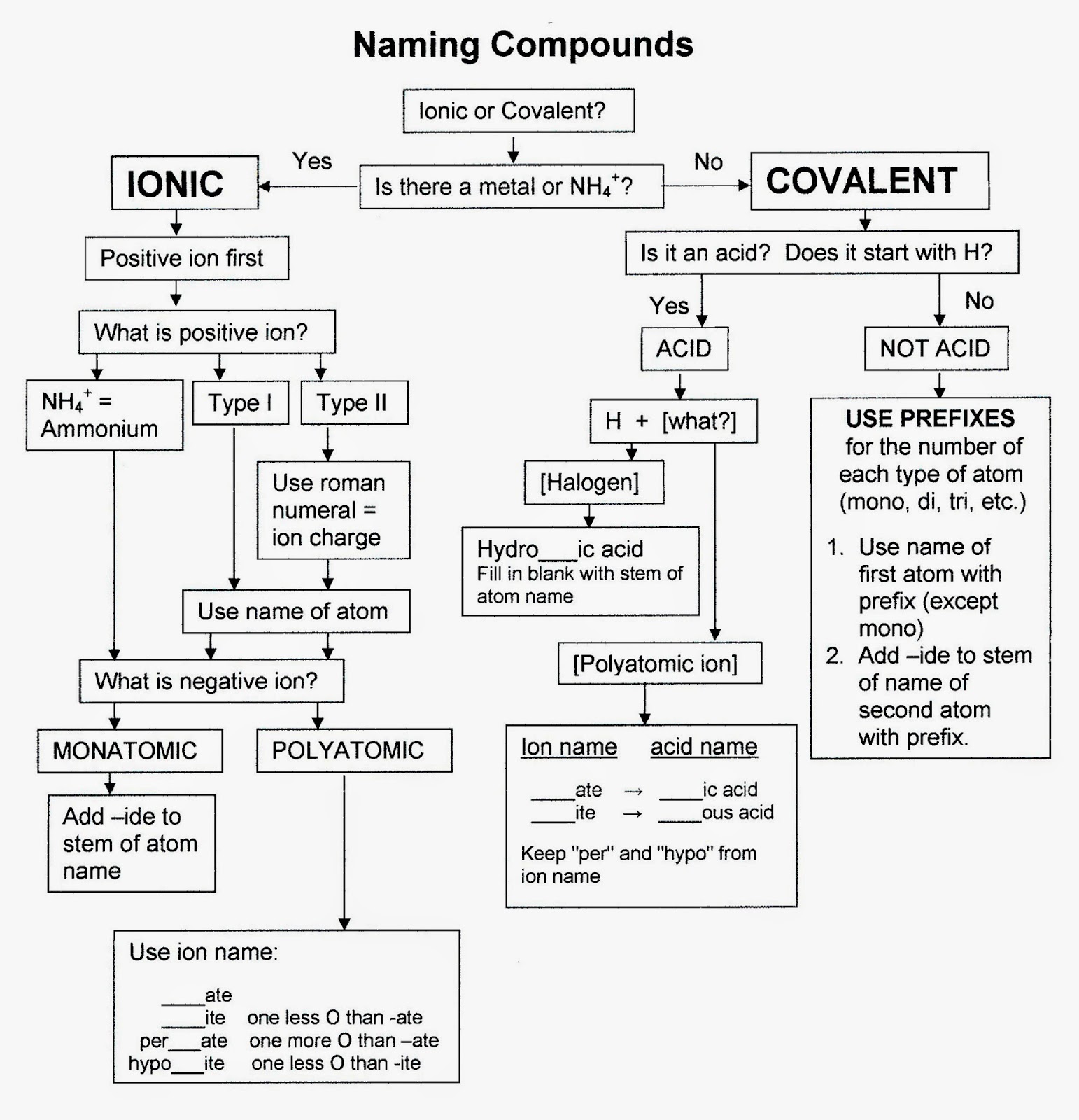
- Chemistry Nomenclature Naming Compounds Flowchart
- Writing Ionic Compound Formula Worksheet Answers
- Mixed Ionic Covalent Compound Naming Answers
- Writing Formulas Criss Cross Method Worksheet Answers
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is a binary compound?
A binary compound is a chemical compound composed of two different elements. This type of compound is formed through a chemical reaction where atoms of two different elements combine in definite proportions. Binary compounds can include both ionic compounds, where electrons are transferred between atoms, and covalent compounds, where electrons are shared between atoms.
How are binary compounds formed?
Binary compounds are formed when two different elements combine chemically to create a compound with a fixed ratio of elements. This bonding occurs through the transfer or sharing of electrons between the atoms, leading to the formation of chemical bonds such as ionic, covalent, or metallic bonds, depending on the elements involved. The resulting compound has distinct properties from the elements it is composed of, and its formula is generally represented with the simplest whole number ratio of the elements involved.
What are the main rules for naming binary compounds?
The main rules for naming binary compounds include identifying the type of elements present (metal or non-metal), naming the cation (metal) first followed by the anion (non-metal) with the suffix -ide, using prefixes to indicate the number of each element present if more than one, and always maintaining the overall charge balance of the compound. Additionally, Roman numerals may be used in the cation name to indicate the charge of transition metals with variable charges.
How do you determine the formula of a binary compound?
To determine the formula of a binary compound, you need to identify the chemical symbols of the elements involved and their respective charges. Then, you balance the charges to create a neutral compound by either crisscrossing the charges or using the crisscross method. For example, if one element has a +2 charge and the other has a -1 charge, the formula would be written as the element with -1 charge followed by a subscript 2 and then the element with +2 charge. This method helps you determine the correct ratio of elements in the compound.
Give an example of a binary compound with a metal and a nonmetal.
An example of a binary compound with a metal and a nonmetal is sodium chloride, which is commonly known as table salt. Sodium (Na) is the metal in this compound, while chlorine (Cl) is the nonmetal. Sodium chloride is formed by the reaction between the metal sodium and the nonmetal chlorine to create a stable ionic compound.
Give an example of a binary compound with two nonmetals.
An example of a binary compound with two nonmetals is carbon dioxide (CO2). This compound is formed by carbon, a nonmetal, bonding with two oxygen atoms, also nonmetals, through covalent bonds.
How are the charges of the ions determined in a binary compound?
The charges of ions in a binary compound are determined based on the number of electrons that atoms gain or lose in order to achieve a stable electron configuration. Typically, metals lose electrons to form positively charged cations, while nonmetals gain electrons to form negatively charged anions. The charges on the ions are determined by the ratio of the positive and negative charges needed to balance each other in the compound, following the principle of charge neutrality.
What is the significance of the Roman numerals in the name of a binary compound?
The Roman numerals in the name of a binary compound indicate the charge of the cation in the compound. The Roman numeral tells us the oxidation state of the metal ion since some metals can have multiple oxidation states. This helps to properly identify and name the compound, as the charge of the cation influences the ratio of the ions in the compound and is important for balancing the overall charge in the chemical formula.
How does the number of atoms in a binary compound affect its formula?
The number of atoms in a binary compound directly influences its formula through the ratio of the elements present. For example, in a compound such as water (H2O), the 2 in front of the hydrogen (H) indicates that there are two hydrogen atoms for every one oxygen (O) atom. This ratio is essential in determining the correct formula of the compound and reflects the specific combination of elements present in the compound.
How can you determine the molar mass of a binary compound?
To determine the molar mass of a binary compound, you need to add the atomic masses of all the atoms in the compound. This can be calculated by multiplying the atomic mass of each element by the number of atoms present in the compound and then summing these values together. The resulting sum is the molar mass of the binary compound, expressed in grams per mole.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.

























Comments