Writing and Naming Ionic Compounds Worksheet
Are you struggling to understand the concept of writing and naming ionic compounds? Look no further! This worksheet is designed to help you grasp the fundamentals of this topic and master the art of correctly identifying the entity and subject involved. Whether you are a high school student studying chemistry for the first time or a college student looking to brush up on your skills, this worksheet is suitable for anyone seeking a solid foundation in writing and naming ionic compounds.
Table of Images 👆
- Naming Ionic Compounds Worksheet Answer Key
- Writing Formulas for Binary Ionic Compounds Worksheets
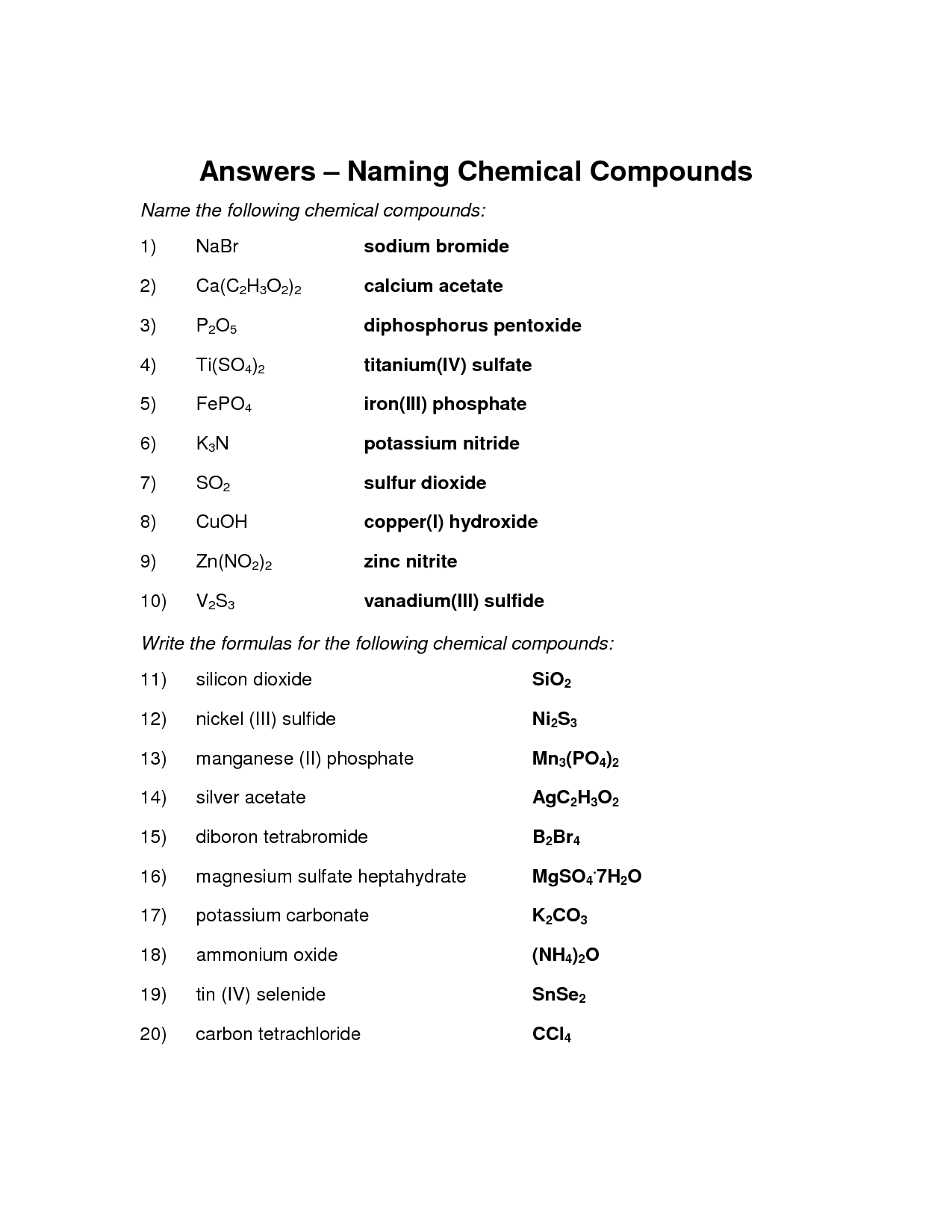
- Naming Chemical Compounds Worksheet Answers
- Naming Binary Ionic Compounds Worksheet Answers
- Writing Chemical Formula Examples
- Naming Compounds Worksheet
- Naming Acids and Bases Worksheet
- Naming Ionic Compounds Worksheet Answers
- Compounds and Covalent Naming Worksheet
- Roman Numerals Worksheets
- Ionic and Covalent Bond Practice
- Hydrates and Nomenclature Worksheet Answers
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is the purpose of writing and naming ionic compounds?
The purpose of writing and naming ionic compounds is to systematically identify and represent the chemical composition of substances that are made up of positively and negatively charged ions. By following specific naming conventions, it becomes easier to communicate and understand the specific elements involved in the compound, their chemical formula, and overall structure, facilitating scientific communication and ensuring consistency in chemical nomenclature.
How do you determine the formula for an ionic compound?
To determine the formula for an ionic compound, you first need to identify the ions present in the compound. Then, you balance the charges of the ions to determine the ratio of positive to negative ions that will result in a neutral compound. This ratio becomes the chemical formula of the ionic compound.
What are cations and anions in ionic compounds?
Cations are positively charged ions in ionic compounds, formed when an atom loses electrons. Anions, on the other hand, are negatively charged ions in ionic compounds, formed when an atom gains electrons. In an ionic compound, cations and anions are attracted to each other through electrostatic forces, creating a stable structure.
How do you name a cation?
To name a cation, you simply write the name of the element followed by the word "ion" unless the element is a metal with a variable charge, in which case you would indicate the charge as a Roman numeral in parentheses after the element name. For example, the cation of sodium would be named "sodium ion," while the cation of iron(III) would be named "iron(III) ion.
How do you name a monatomic anion?
To name a monatomic anion, you use the root of the element name with the suffix "-ide" added at the end. For example, a chlorine atom that gains an extra electron to become an anion is named chloride.
How do you name a polyatomic ion?
To name a polyatomic ion, you typically follow the rules for naming ions. The name usually consists of the name of the main element in the ion, followed by the suffix "-ate" or "-ite" depending on the number of oxygen atoms present in the ion. If there are more oxygen atoms, the suffix "-ate" is used, and if there are fewer oxygen atoms, the suffix "-ite" is used. Additionally, if there is one less oxygen than the "-ite" ion, the prefix "hypo-" is added before the main element name, and if there is one more oxygen than the "-ate" ion, the prefix "per-" is added.
How do you write the formula for an ionic compound given the names of the ions?
To write the formula for an ionic compound given the names of the ions, you need to determine the charges of the ions involved. Once you know the charges, you can balance them to create a neutral compound. The formula will have the cation listed first followed by the anion, and the subscripts will be adjusted based on the charges of the ions to ensure electrical neutrality in the compound.
How do you name an ionic compound given the formula?
To name an ionic compound given the formula, you must first identify the cation and anion present. The cation (positively charged ion) is typically listed first in the formula, followed by the anion (negatively charged ion). Once you have identified the ions, you can use the naming conventions for ionic compounds – the cation retains its original name, while the anion's name is modified to end in "-ide." Simply combine the names of the cation and anion to form the name of the ionic compound.
What are some common naming patterns for ionic compounds?
Some common naming patterns for ionic compounds include using the cation's name first followed by the anion's name, modifying the anion's ending to "-ide" if it is an element, using Roman numerals to indicate the charge of the cation if it can vary, and using prefixes like "bi-", "tri-", or "tetra-" to indicate multiple ions in the formula.
How do you indicate the charge of an ion in a compound's name?
To indicate the charge of an ion in a compound's name, you use Roman numerals enclosed in parentheses after the metal ion's name. The Roman numeral represents the charge on the metal ion in the compound. This naming convention is particularly important for transition metals that can exhibit multiple oxidation states.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.





























Comments