Types of Chemical Reactions Worksheet
Chemical reactions are a fundamental concept in chemistry, and understanding the different types of reactions is crucial for students studying this subject. Whether you are a high school student learning the basics or a college student seeking to deepen your knowledge, a types of chemical reactions worksheet can be a valuable tool in solidifying your understanding of these concepts.
Table of Images 👆
- Types Chemical Reactions Worksheets Answers
- Chemical Reaction Types Worksheet
- Classifying Chemical Reactions Worksheet
- Identifying Chemical Reactions Worksheet
- Balancing Chemical Reactions Worksheet
- Double Replacement Reaction Worksheet Answers
- Balancing Chemical Equations Worksheet Answers
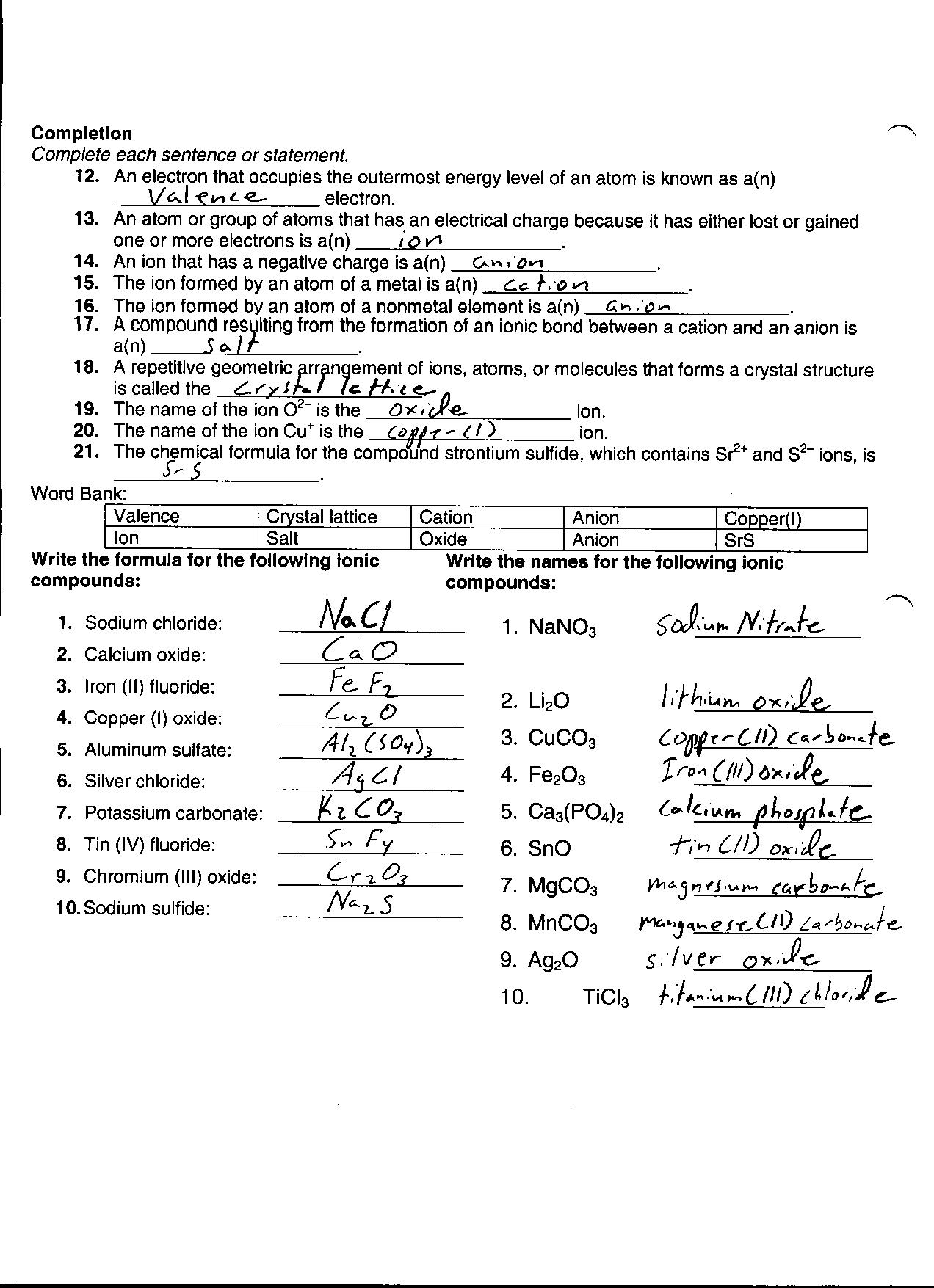
- Chemical Reactions Worksheet Answer Key
- Mole Calculation Worksheet Answer Key
- Activity Series Chemistry POGIL Answer Keys
- Chemistry Double Replacement Reaction Worksheet
- Ionic Reactions in Aqueous Solutions Lab
- Decomposition and Synthesis Reactions Worksheet Answers
- Balancing Equations Worksheet Answer Key
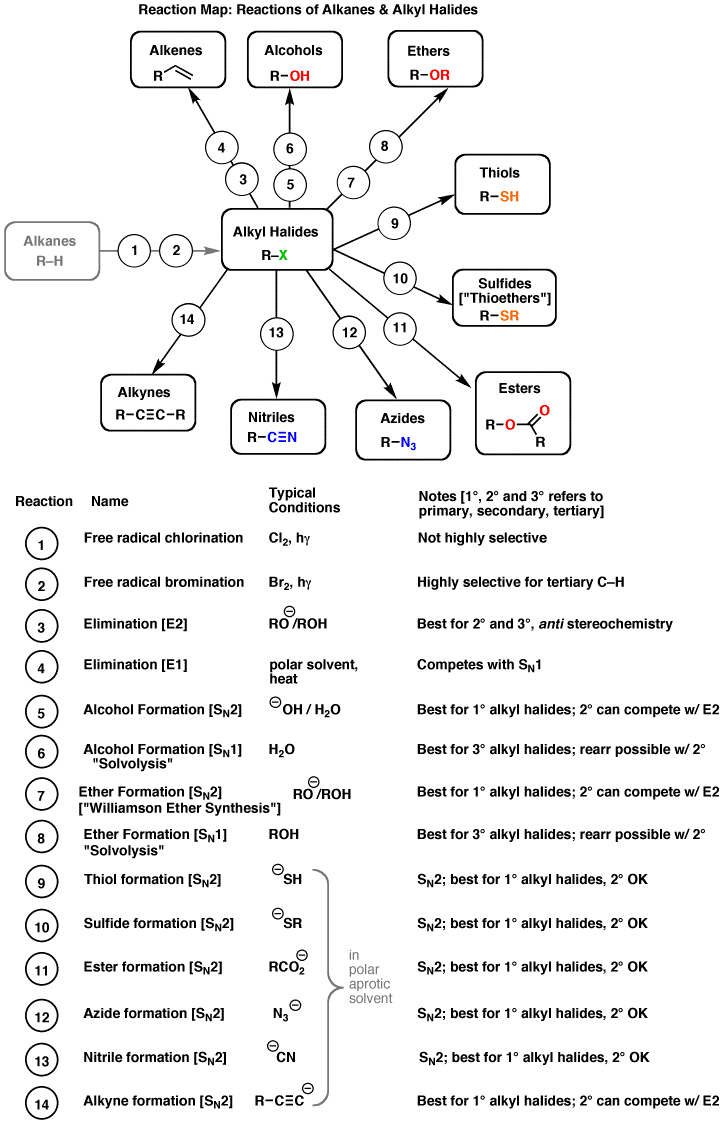
- Organic Chemistry Reaction Map
- Chemical Reaction Rate Graph
- Specific Heat Worksheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is a synthesis reaction?
A synthesis reaction is a chemical reaction where two or more simple substances combine to form a more complex product. This type of reaction typically involves the formation of a single compound from its constituent elements or simpler compounds, often requiring energy input to proceed.
What is a decomposition reaction?
A decomposition reaction is a type of chemical reaction where a single compound breaks down into two or more simpler substances. This process is typically driven by heat, light, or the introduction of a catalyst, resulting in the decomposition of the original compound into its constituent elements or other compounds.
What is a combustion reaction?
A combustion reaction is a type of chemical reaction where a substance combines with oxygen gas to produce heat, light, and usually carbon dioxide and water as products. This reaction is typically rapid and exothermic, generating a significant amount of heat energy. Combustion reactions are commonly seen in everyday life, such as in burning wood for fuel or in the combustion engines of cars.
What is a single replacement reaction?
A single replacement reaction is a type of chemical reaction where one element replaces another element in a compound. It occurs when a more reactive element displaces a less reactive element in a compound, resulting in the formation of a new compound and a different element.
What is a double replacement reaction?
A double replacement reaction, also known as a double displacement reaction, is a chemical reaction where two compounds react by exchanging ions to form two new compounds. In this reaction, the positive and negative ions in the compounds switch places to produce two new compounds.
What is a precipitation reaction?
A precipitation reaction is a type of chemical reaction in which two soluble compounds react to form an insoluble solid compound, called a precipitate. This solid compound is formed when the products of the reaction are not soluble in the solvent and therefore separate out as a solid. This reaction can be used to selectively separate ions in a mixture based on their solubility properties.
What is an acid-base reaction?
An acid-base reaction is a chemical reaction that occurs when an acid and a base interact with each other to form water and a salt. Acids donate protons (H+ ions) while bases accept protons, leading to the formation of water and a salt as the end products of the reaction. This type of reaction plays a significant role in various chemical processes and is commonly studied in chemistry.
What is a redox reaction?
A redox reaction is a chemical reaction in which there is a transfer of electrons between reactant species. One reactant loses electrons (oxidation) while the other gains electrons (reduction). The word "redox" comes from the combination of reduction and oxidation processes occurring simultaneously in these reactions.
What is a neutralization reaction?
A neutralization reaction is a chemical reaction between an acid and a base that results in the formation of water and a salt. The acid donates a hydrogen ion (H+) and the base donates a hydroxide ion (OH-) to form water, while the remaining ions combine to form a salt. This reaction helps to balance the pH of a solution by neutralizing the acidic or basic properties of the substances involved.
What is a displacement reaction?
A displacement reaction is a chemical reaction in which a more reactive element displaces a less reactive element from a compound. This results in the more reactive element taking the place of the less reactive element, causing a change in the chemical composition of the compound.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



































Comments