Thermodynamics Practice Worksheet Answers
In this blog post, we will be discussing the importance of worksheets for individuals studying thermodynamics. Worksheets are an essential tool for those who are looking to enhance their understanding of this subject by providing practice problems and exercises specifically tailored to reinforce key concepts and calculations.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
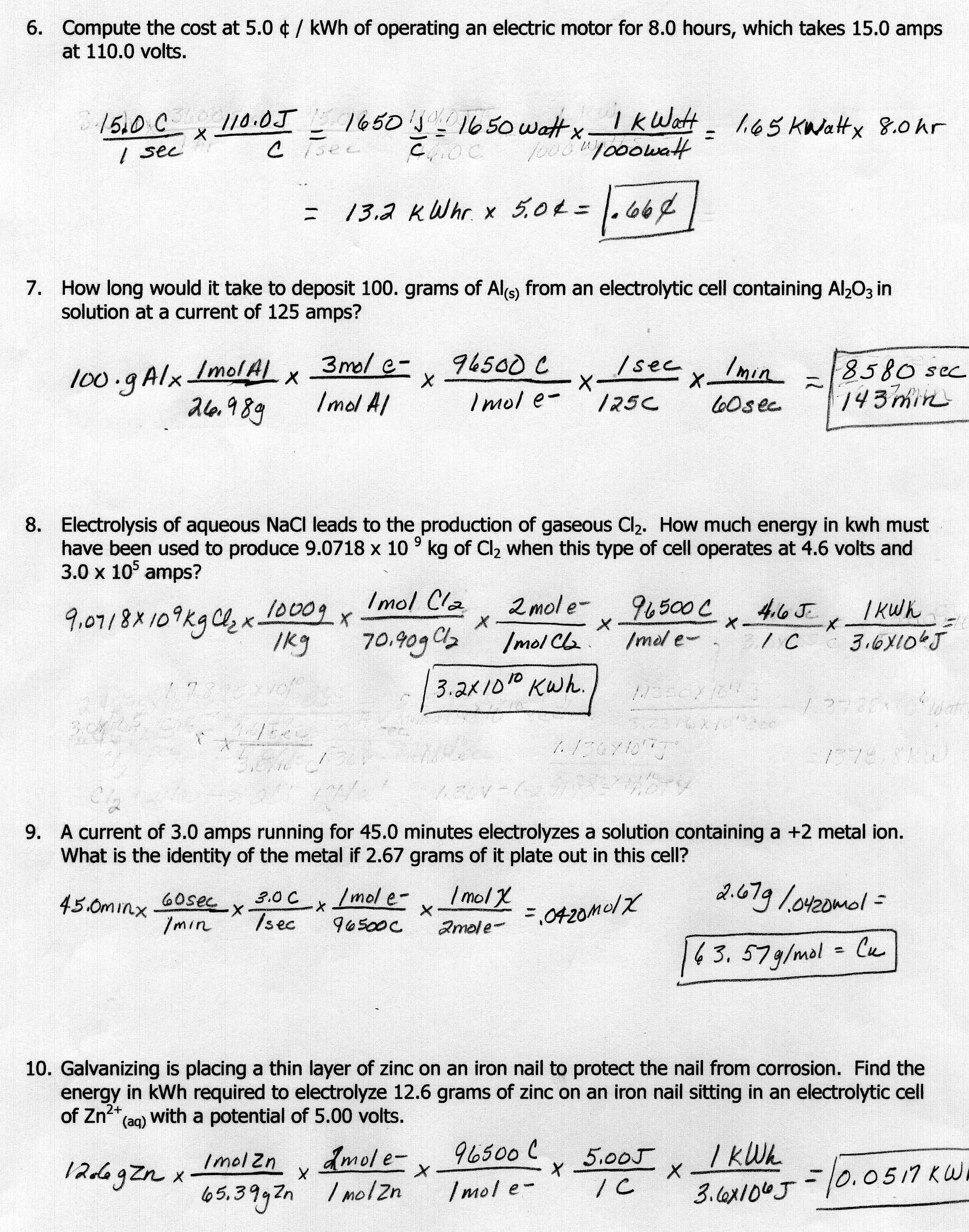
What is the first law of thermodynamics?
The first law of thermodynamics states that energy cannot be created or destroyed in an isolated system; it can only change forms. This principle is also known as the law of conservation of energy, which emphasizes that the total amount of energy within a closed system remains constant over time, regardless of the changes or transformations it undergoes.
How does the second law of thermodynamics relate to entropy?
The second law of thermodynamics states that in a closed system, entropy tends to increase over time. Entropy is a measure of the disorder or randomness of a system, and the second law essentially implies that systems naturally move towards a state of greater disorder. As entropy increases, the amount of energy available for useful work decreases, leading to less efficiency and more randomness in the system. Thus, the second law of thermodynamics and entropy are intimately related in describing the spontaneous direction of natural processes towards increased disorder in closed systems.
Explain the concept of heat transfer in thermodynamics.
Heat transfer is the process by which thermal energy is exchanged between two or more systems due to a temperature difference. In thermodynamics, this transfer can occur through three main mechanisms: conduction, convection, and radiation. Conduction involves the direct transfer of heat through a material, while convection involves the transfer of heat through the movement of fluids. Radiation, on the other hand, occurs through electromagnetic waves. The direction of heat transfer is always from the hotter object to the cooler object, in order to establish thermal equilibrium between the systems involved.
How is internal energy defined in thermodynamics?
Internal energy in thermodynamics is defined as the total energy contained within a system, including the kinetic and potential energies of its particles. It is a sum of the system's microscopic energy components, such as the internal vibrations, rotations, and translations of its molecules and atoms. Internal energy does not include the energy associated with the system's motion or position in space, but rather focuses on the energy stored within the system itself.
What is the difference between an open, closed, and isolated system in thermodynamics?
In thermodynamics, an open system allows both energy and matter to be exchanged with the surroundings, a closed system only allows energy exchange with the surroundings but not matter, while an isolated system does not allow either energy or matter exchange with the surroundings. This distinction is important in studying how systems respond to changes in their surroundings and the conservation of energy and mass within the system.
Describe the process of adiabatic expansion.
Adiabatic expansion is a thermodynamic process in which a gas expands in a way that no heat is exchanged with its surroundings. As the gas expands, it does work on its surroundings, causing a decrease in its internal energy and temperature. This decrease in temperature results in a decrease in pressure and volume of the gas, while its entropy remains constant. Adiabatic expansion is often seen in natural phenomena like the rising of a parcel of air in the atmosphere or in industrial applications like compressed air systems.
How does the Carnot cycle illustrate the concept of thermal efficiency?
The Carnot cycle illustrates the concept of thermal efficiency by showing the maximum theoretical efficiency that can be achieved for a heat engine operating between two temperature reservoirs. The cycle demonstrates that the efficiency of an engine is determined by the temperatures at which heat is added and removed, with the highest efficiency occurring when the engine operates reversibly and isothermaly, following the Carnot principles. This ideal cycle helps to highlight that real-world engines will always have inefficiencies due to factors such as friction and heat losses, causing their actual efficiency to be lower than the Carnot efficiency.
Explain the role of enthalpy in thermodynamics.
Enthalpy is a fundamental concept in thermodynamics, representing the total energy of a system. It accounts for both the internal energy of a system and the energy required to maintain constant pressure during a process. Enthalpy is crucial in determining the heat transfer that occurs in chemical reactions and phase changes, as well as in understanding the overall energy balance of a system. Changes in enthalpy help to predict the direction of a chemical reaction and the amount of heat involved. In essence, enthalpy is a key parameter in studying the energy changes that take place in various thermodynamic processes.
What is the meaning of the term "latent heat" in thermodynamics?
In thermodynamics, "latent heat" refers to the heat energy released or absorbed by a substance during a phase change, such as from solid to liquid or liquid to gas, without a change in temperature. This energy is used to break or form intermolecular bonds within the substance, resulting in a change of state rather than a change in temperature.
Describe the significance of the triple point of a substance in thermodynamics.
The triple point of a substance in thermodynamics is a critical point where the three phases of a substance (solid, liquid, gas) coexist in equilibrium. This point is significant because it represents a unique set of conditions where the substance has a specific pressure and temperature at which all three phases can exist in harmony. It is a pivotal point for accurately defining the phase diagram of a substance and provides essential information for determining the specific conditions under which a substance can transition between phases.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.






















Comments