Thermal Energy and Temperature Worksheet
Are you a science teacher or a homeschooling parent looking for a comprehensive worksheet on thermal energy and temperature? Look no further! This thermal energy and temperature worksheet is designed to provide your students with an in-depth understanding of these concepts. With a focus on clear explanations and engaging activities, this worksheet will help your students grasp the fundamental principles of thermal energy and temperature.
Table of Images 👆
More Energy Worksheets
Light and Heat Energy WorksheetsTypes of Energy Transfer Worksheet
Energy Light Heat Sound Worksheets
3 Forms of Energy Worksheets
Energy Worksheets for Third Grade
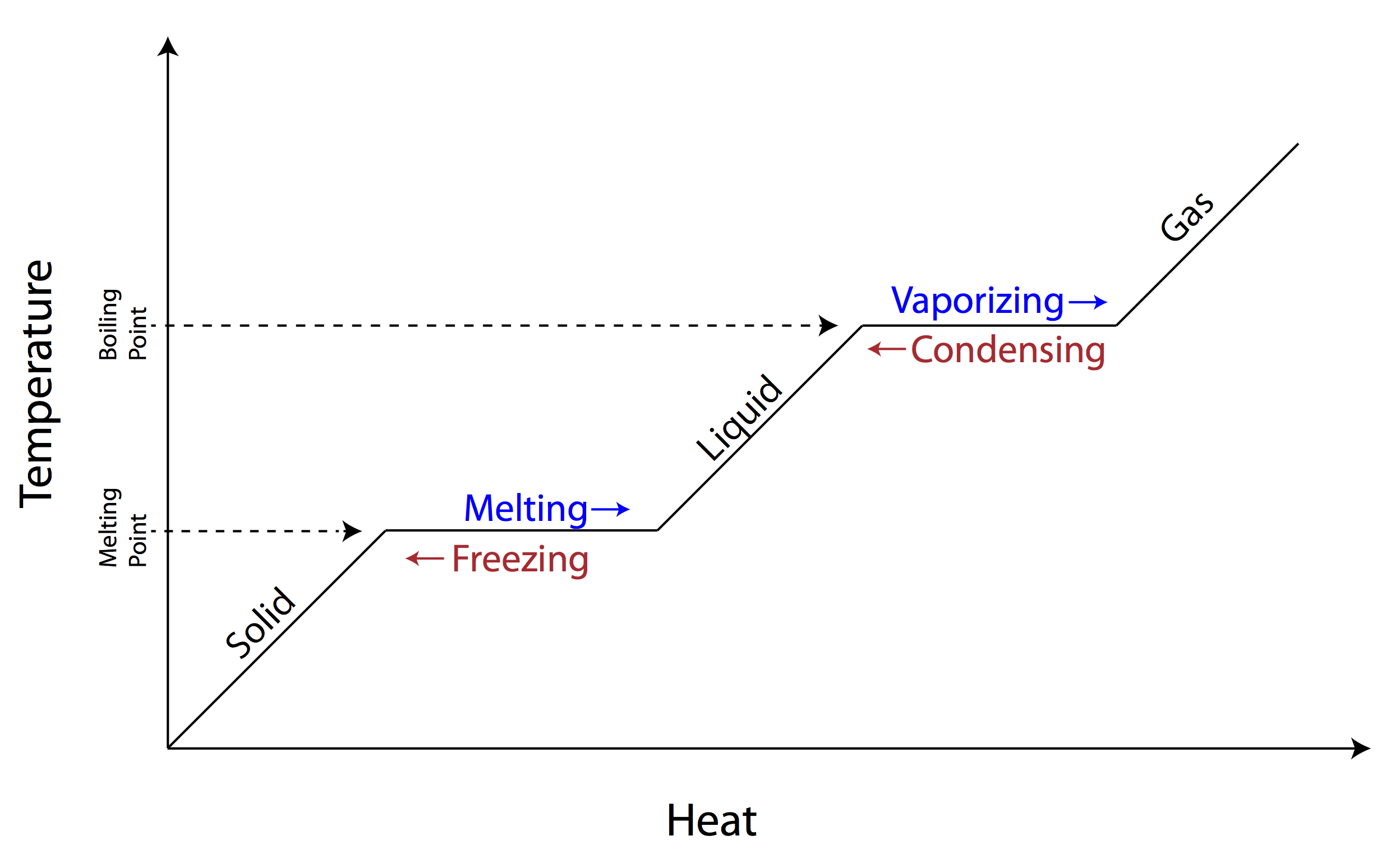
What is thermal energy?
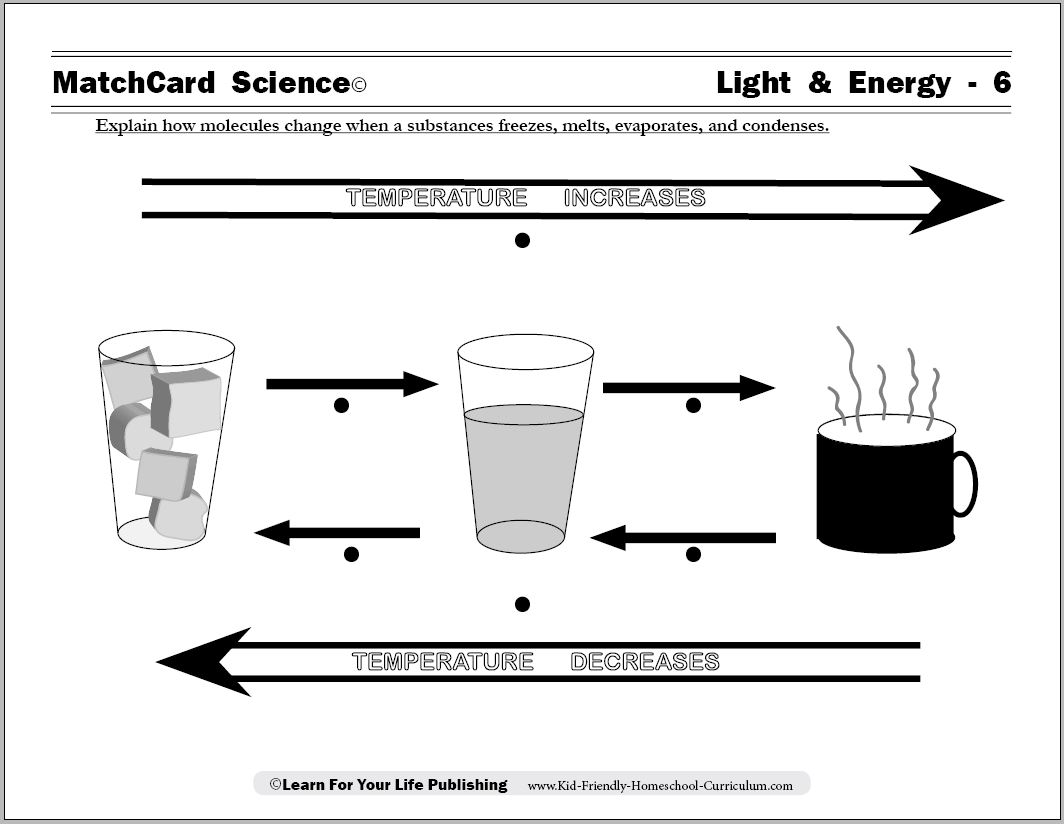
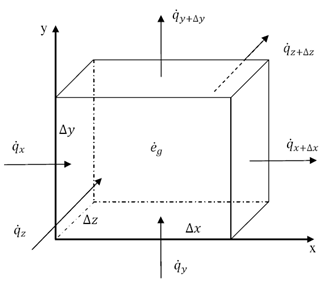
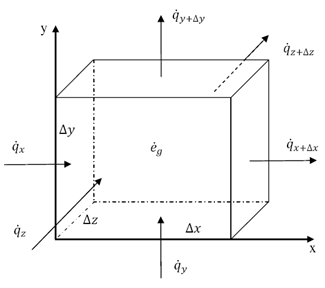
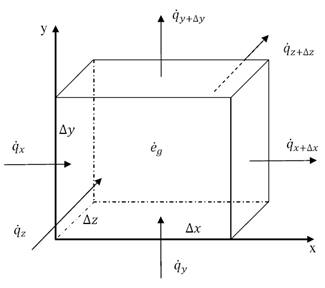
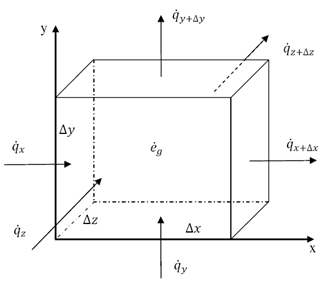
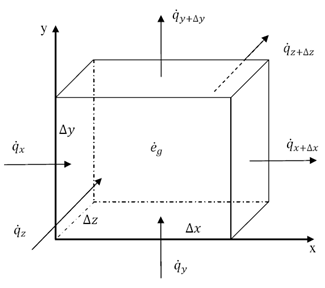
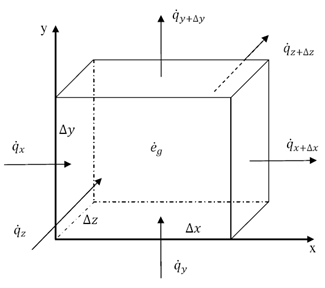
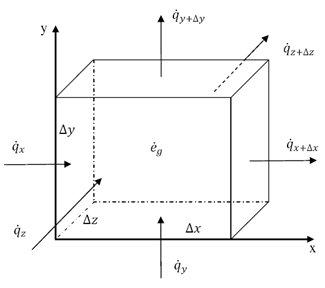
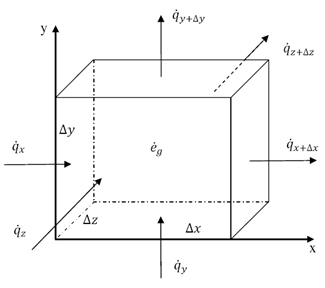
Thermal energy is the internal energy of a system due to the kinetic energy of its atoms and molecules. It is associated with the temperature of the system and is a form of energy that can be transferred between objects through heat transfer mechanisms such as conduction, convection, and radiation. Thermal energy plays a crucial role in many natural phenomena and technological processes.
How does temperature differ from thermal energy?
Temperature is a measure of the average kinetic energy of particles in a substance, whereas thermal energy is the total energy of all the particles in a substance due to their motion. Temperature is a specific measure of the hotness or coldness of an object, while thermal energy encompasses both the kinetic and potential energies of the particles in a substance. Temperature does not depend on the size or mass of the object, while thermal energy does.
What is the relationship between temperature and kinetic energy?
Temperature is directly related to the average kinetic energy of particles in a substance. As temperature increases, the particles within the substance gain more energy, resulting in higher kinetic energy. Likewise, as temperature decreases, the particles have less energy and therefore lower kinetic energy. This relationship between temperature and kinetic energy is a fundamental concept in understanding the behavior of matter.
How is thermal energy transferred between objects?
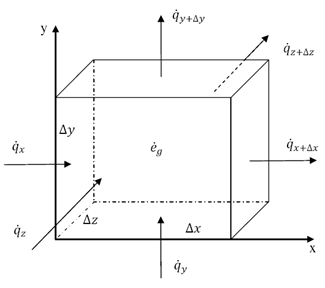
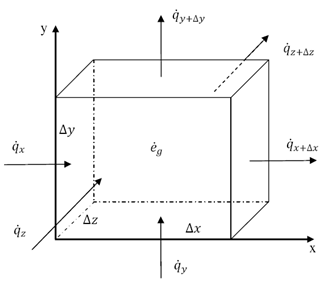
Thermal energy can be transferred between objects through three main mechanisms: conduction, convection, and radiation. Conduction occurs when heat is transferred through direct contact between materials, such as touching a hot stove. Convection involves the transfer of heat through the movement of fluids, such as air or water, carrying heat energy from one place to another. Radiation is the transfer of heat through electromagnetic waves, like how the sun's rays warm the Earth. These three mechanisms work together to ensure that thermal energy is distributed and balanced in our environment.
What is the specific heat capacity of a substance?
The specific heat capacity of a substance is the amount of heat energy required to raise the temperature of one unit mass of the substance by one degree Celsius (or Kelvin) without a phase change occurring. It is a characteristic property of a substance and is typically expressed in units of J/(g°C) or J/(g*K).
What is conduction and how does it transfer thermal energy?
Conduction is the process through which thermal energy is transferred when two objects at different temperatures come into direct contact with each other. Heat is transferred from the hotter object to the cooler one through the collision of particles within the materials. The vibrating particles in the hotter object transfer kinetic energy to the particles in the cooler object, causing them to increase in motion and temperature until equilibrium is reached between the two objects.
Describe convection and how it affects thermal energy transfer.
Convection is a process of heat transfer caused by the movement of fluids, such as liquids or gases, due to temperature differences within the fluid. As a fluid is heated, it becomes less dense and rises, while cooler, denser fluid sinks. This movement creates a convection current, transferring thermal energy from one area to another. In this way, convection plays a significant role in redistributing heat in fluids and transferring thermal energy from hot to cold regions.
How does radiation transfer thermal energy?
Radiation transfers thermal energy through electromagnetic waves or particles moving through space. The emitted radiation carries heat energy away from a hotter object to a cooler one, resulting in a transfer of thermal energy. This form of heat transfer does not require a medium for the transfer to occur, making it effective in vacuum environments.
Explain the concept of thermal equilibrium.
Thermal equilibrium is a state in which two systems have reached a balance in temperature, meaning there is no net flow of heat between them. In this state, the temperatures of the systems are equal, and they are said to be in thermal equilibrium because there is no further exchange of heat occurring. This concept is a fundamental aspect of thermodynamics and is crucial in understanding how heat energy moves and distributes in different systems.
How does insulation affect the transfer of thermal energy?
Insulation reduces the transfer of thermal energy by creating a barrier that blocks the flow of heat between two objects or areas with different temperatures. It works by trapping air pockets within its material, which slows down the transfer of heat through conduction, convection, and radiation. This helps to maintain a consistent temperature inside a building, preventing heat loss in cold weather and heat gain in hot weather, ultimately reducing the need for heating and cooling systems.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.




















Comments