Temperature and Thermal Energy Worksheet
Are you a science teacher looking for engaging worksheets to help your students understand concepts related to temperature and thermal energy? You've come to the right place. Our Temperature and Thermal Energy Worksheet is specifically designed to make learning about these topics easy and accessible for middle school and high school students. In this blog post, we will provide an overview of the worksheet and how it can benefit your students' understanding of temperature and thermal energy.
Table of Images 👆
- Potential and Kinetic Energy Worksheet Answer Key
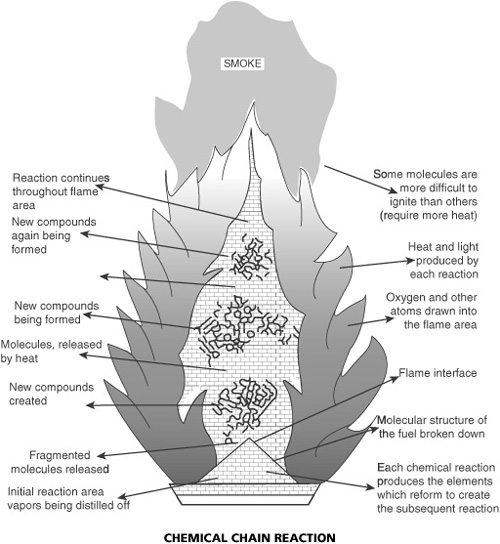
- Fire Tetrahedron Chemical Chain Reaction
- Heat and Thermal Energy Worksheet
- Blank Fill in the Light and Sound Waves Worksheets
- Chapter 8 Covalent Bonding Worksheet Answer Key
- Venn Diagram Compare
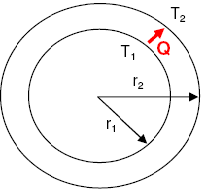
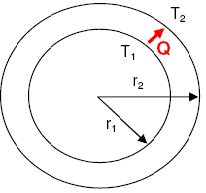
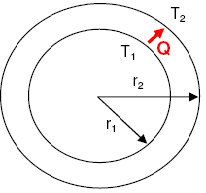
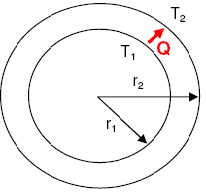
- Temperature Distribution Fin Heat Transfer
- Heating Curve of Water Lab Report
- Conduction Heat Transfer Equation
More Energy Worksheets
Light and Heat Energy WorksheetsTypes of Energy Transfer Worksheet
Energy Light Heat Sound Worksheets
3 Forms of Energy Worksheets
Energy Worksheets for Third Grade
What is the difference between temperature and thermal energy?
Temperature and thermal energy are related concepts but differ in their definitions. Temperature is a measure of the average kinetic energy of the particles in a substance, while thermal energy is the total kinetic energy of all the particles in a substance. In other words, temperature gives an indication of the intensity of heat, while thermal energy reflects the total amount of heat present in a system.
How is temperature measured?
Temperature is measured using a device called a thermometer. Thermometers work by detecting changes in a material's physical properties, such as expansion or contraction in response to temperature fluctuations, and then displaying the measurement on a scale. Common types of thermometers include mercury thermometers, digital thermometers, infrared thermometers, and thermocouples.
What are the three common temperature scales?
The three common temperature scales are Celsius, Fahrenheit, and Kelvin.
How does thermal energy affect the motion of particles?
Thermal energy affects the motion of particles by increasing their kinetic energy, causing them to vibrate and move more rapidly. As particles gain thermal energy, they start to move faster and collide more frequently with each other, leading to increased motion and random movement within a substance. This increased motion is what we perceive as heat in a material, influencing its physical properties and behavior.
What is the relationship between temperature and thermal energy?
Temperature is a measure of the average kinetic energy of the particles in a substance, while thermal energy is the total kinetic energy of all the particles in a substance. The relationship between temperature and thermal energy is that as the temperature of a substance increases, the thermal energy also increases because the particles are moving faster, resulting in a higher total kinetic energy. Conversely, when the temperature decreases, the thermal energy decreases as well because the particles are moving slower, leading to a lower total kinetic energy.
How does temperature affect the volume of most substances?
As temperature increases, the volume of most substances also increases. This is because heating a substance causes its particles to move faster and farther apart, leading to an expansion of the substance. Conversely, as temperature decreases, the volume of most substances decreases because the particles slow down and move closer together, resulting in a reduction in volume.
What is the specific heat capacity of a substance and how does it relate to temperature?
Specific heat capacity is the amount of heat energy required to raise the temperature of one unit mass of a substance by one degree Celsius. It is a constant property for a substance and is dependent on the type of material. As temperature increases, the specific heat capacity of a substance remains constant. However, the amount of energy required to raise the temperature of the substance by one degree Celsius will change as the mass of the substance changes.
How does thermal energy transfer occur?
Thermal energy transfer occurs through conduction, convection, and radiation. In conduction, heat is transferred through direct contact between materials with different temperatures. Convection involves the transfer of heat through the movement of fluids, such as air or water. Radiation transfers heat through electromagnetic waves emitted from a warmer object to a cooler one without the need for a medium. These three methods work together to distribute thermal energy and maintain balance in temperature gradients.
What are the three main methods of heat transfer?
The three main methods of heat transfer are conduction, convection, and radiation. Conduction is the transfer of heat through a material by direct contact, convection is the transfer of heat through fluid movement like air or water, and radiation is the transfer of heat through electromagnetic waves.
How does insulation help regulate temperature and thermal energy transfer?
Insulation helps regulate temperature and thermal energy transfer by acting as a barrier between different environments, preventing the flow of heat between them. Insulation materials have low thermal conductivity, meaning they are poor conductors of heat. This reduces heat transfer through conduction, convection, and radiation, keeping spaces warmer in the winter and cooler in the summer. By limiting the amount of heat that can escape or enter a space, insulation helps maintain a consistent temperature and reduces the need for heating or cooling systems to work harder, ultimately increasing energy efficiency.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.
























Comments