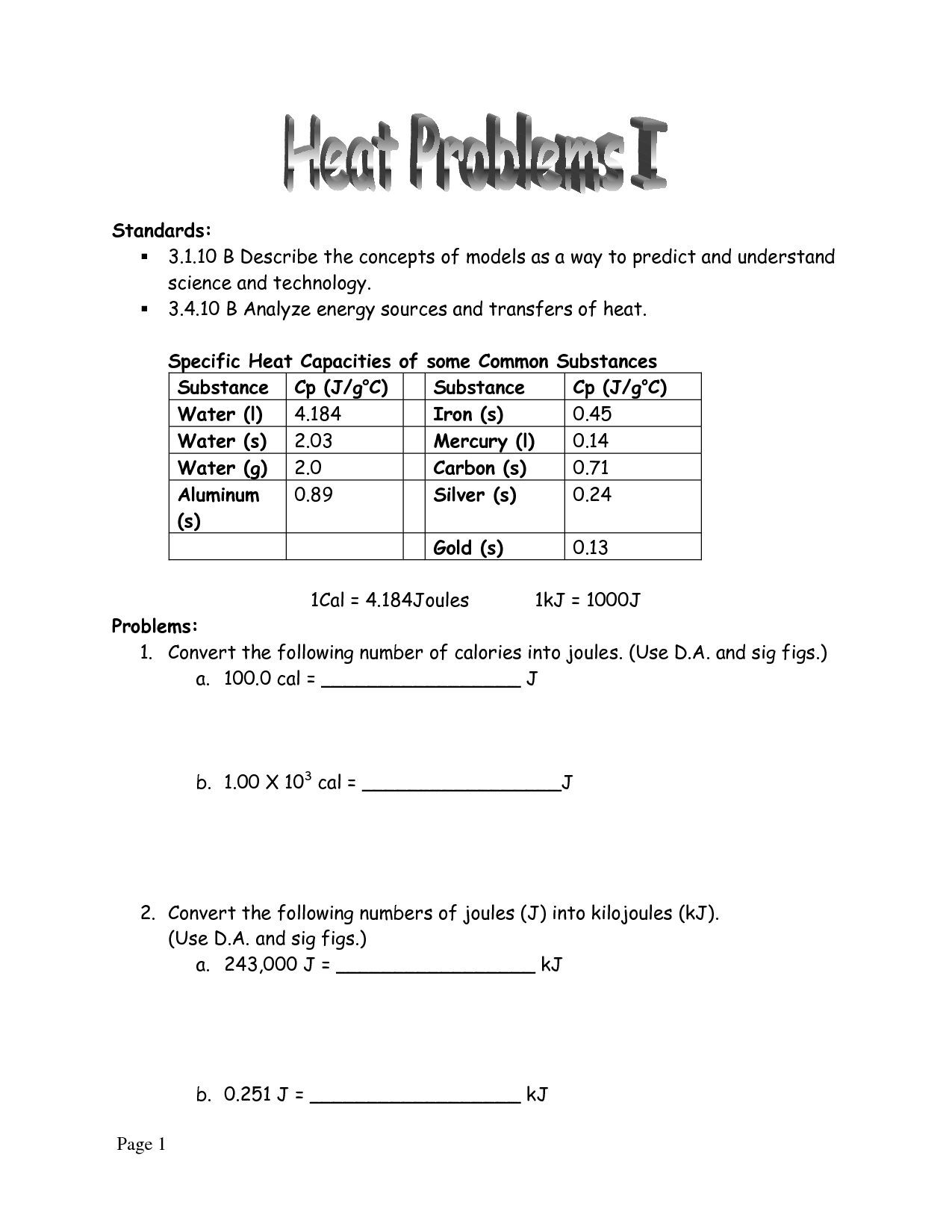
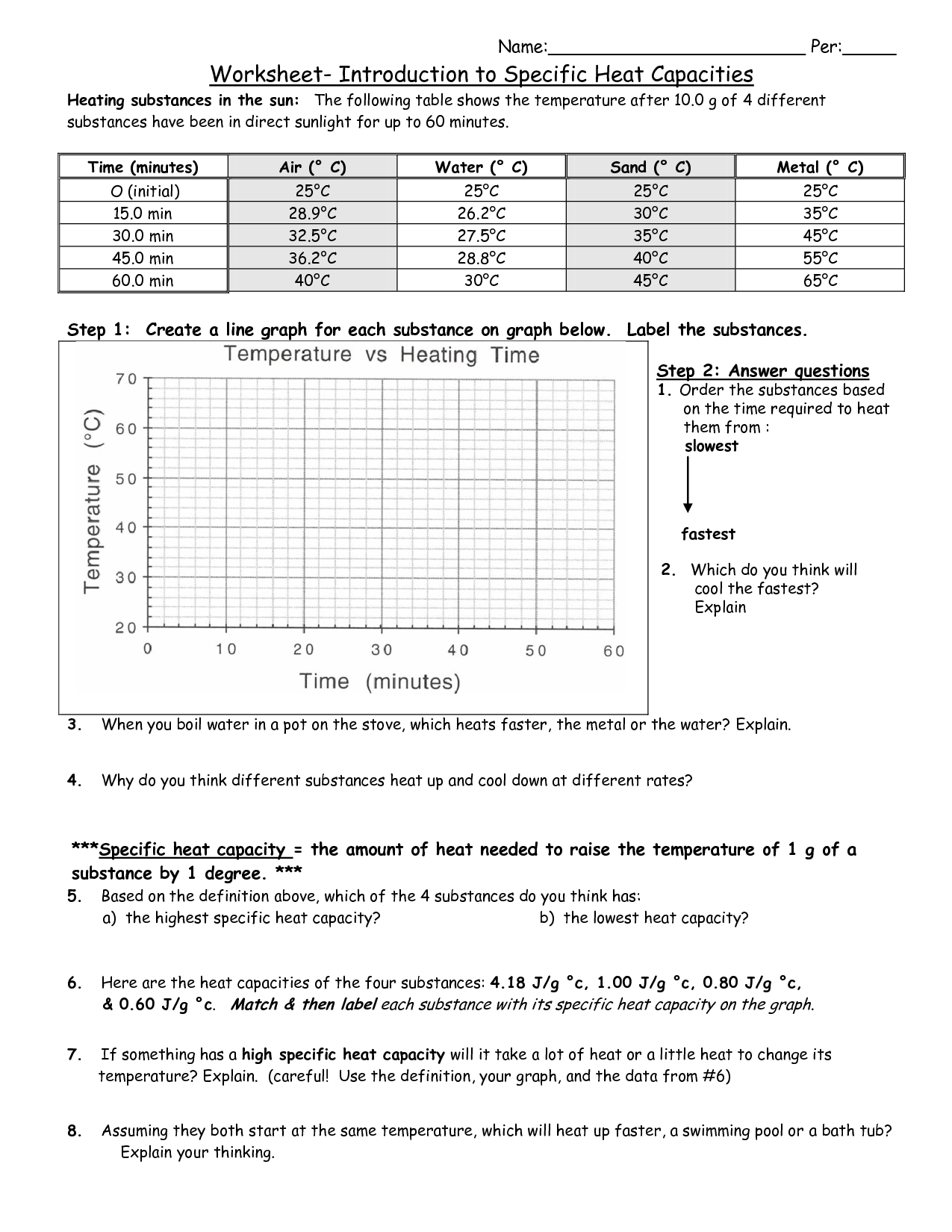
Specific Heat Worksheet
Are you searching for a helpful resource to practice specific heat problems? Look no further than our specific heat worksheet. This worksheet is designed for students in high school or college who are learning about the concept of specific heat and need extra practice with calculations involving this important physical property.
Table of Images 👆
- Specific Heat Calculations Worksheet Answers
- Specific Heat Capacity Worksheet
- Specific Heat Worksheets and Answers
- Specific Heat Practice Problems Worksheet
- Specific Heat Problems Chemistry Worksheet Answers
- Specific Heat Worksheet Answer Key
- Chemistry Specific Heat Worksheet
- For Specific Heat Worksheet Physics
- Specific Heat Capacity Worksheet Answers
- Properties of Water Worksheet Answers
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is specific heat?
Specific heat is the amount of heat required to raise the temperature of one unit mass of a substance by one degree Celsius or Kelvin. It is a physical property that helps determine how much energy is needed to change the temperature of a substance, with different materials having different specific heat values.
How is specific heat measured?
Specific heat is typically measured using a calorimeter, a device designed to accurately measure the heat exchanged during a physical or chemical process. The specific heat capacity of a substance is determined by measuring the change in temperature of a known mass of the substance as it absorbs or releases heat energy. By using the formula Q = mc?T, where Q is the heat energy gained or lost, m is the mass of the substance, c is the specific heat capacity, and ?T is the change in temperature, scientists can calculate the specific heat capacity of a material.
Why is specific heat important in thermodynamics?
Specific heat is important in thermodynamics because it quantifies how much heat energy is required to change the temperature of a substance. It helps in calculating the amount of energy needed for processes such as heating or cooling, determining the thermal efficiency of systems, and understanding the behavior of materials under different temperature conditions. Specific heat also plays a crucial role in designing and optimizing energy systems, as well as in studying heat transfer and thermal equilibrium in various processes.
What is the formula to calculate specific heat?
The formula to calculate specific heat capacity is Q = mc?T, where Q represents the amount of heat energy transferred, m is the mass of the substance, c is the specific heat capacity of the material, and ?T is the change in temperature.
What are the units for specific heat?
The units for specific heat are typically J/(kg·K) or J/(g·°C), representing the amount of heat energy required to raise the temperature of one unit of mass (either one kilogram or one gram) of a substance by one degree Kelvin or Celsius.
How does specific heat differ for different substances?
Specific heat is a physical property that measures the amount of heat energy required to raise the temperature of a unit mass of a substance by one degree Celsius. Different substances have different specific heat capacities due to variations in their molecular structures and compositions. For example, water has a relatively high specific heat capacity compared to most substances, meaning it can absorb and store more heat energy before its temperature increases. This is why water is often used as a coolant. In contrast, substances with lower specific heat capacities, like metals, heat up and cool down quickly, making them good conductors of heat.
How does specific heat affect the temperature change of a substance?
Specific heat is a measure of how much heat energy is needed to raise the temperature of a substance by a certain amount. The higher the specific heat of a substance, the more heat energy is required to cause a temperature change. Therefore, substances with higher specific heat capacities will experience smaller temperature changes when heated or cooled, while substances with lower specific heat capacities will experience larger temperature changes for the same amount of heat added or removed.
How is specific heat related to heat transfer?
Specific heat is a measure of how much heat energy is required to raise the temperature of a substance by a certain amount. It is related to heat transfer because it dictates how effectively a substance can absorb or release heat without experiencing significant changes in temperature. Substances with higher specific heat require more heat energy to change their temperature, making them better at storing or releasing heat. This property influences the rate and amount of heat transfer in a system, as substances with lower specific heat can change temperature more easily and therefore transfer heat more rapidly.
What are some real-life applications of specific heat?
Specific heat is used in various real-life applications, such as in determining the heating and cooling requirements for buildings, designing thermal protection systems for spacecraft, calculating the energy requirement for cooking food, developing temperature control systems in manufacturing processes, and designing equipment such as heat exchangers and refrigeration units. Additionally, specific heat is also utilized in the fields of chemistry and biology for understanding metabolic processes and studying the thermal properties of materials.
How can specific heat be used to determine the heat capacity of a substance?
To determine the heat capacity of a substance using specific heat, you can use the formula Q = mc?T, where Q is the amount of heat transferred, m is the mass of the substance, c is the specific heat of the substance, and ?T is the change in temperature. By measuring the amount of heat transferred when a substance experiences a temperature change, and knowing the mass and specific heat of the substance, you can calculate the heat capacity, which is the amount of heat required to raise the temperature of the substance by a certain amount.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.































Comments