Specific Heat Worksheet Answer Key
Are you struggling to find an answer key for your specific heat worksheet? Look no further! In this blog post, we have compiled a comprehensive answer key for your specific heat worksheet. Whether you are a student studying physics or a teacher looking for a resource to help your students understand the concept of specific heat, this answer key is designed to provide clear and accurate solutions to all the problems in your worksheet. So, let's delve into the world of specific heat and unravel the answers together!
Table of Images 👆
- Specific Heat Worksheet Answers
- Specific Heat Practice Problems Worksheet Answers
- Specific Heat Worksheets and Answers
- Specific Heat Capacity Worksheet Answers
- Stoichiometry Practice Worksheet Answer Key
- Heat Transfer Worksheet Answer Key
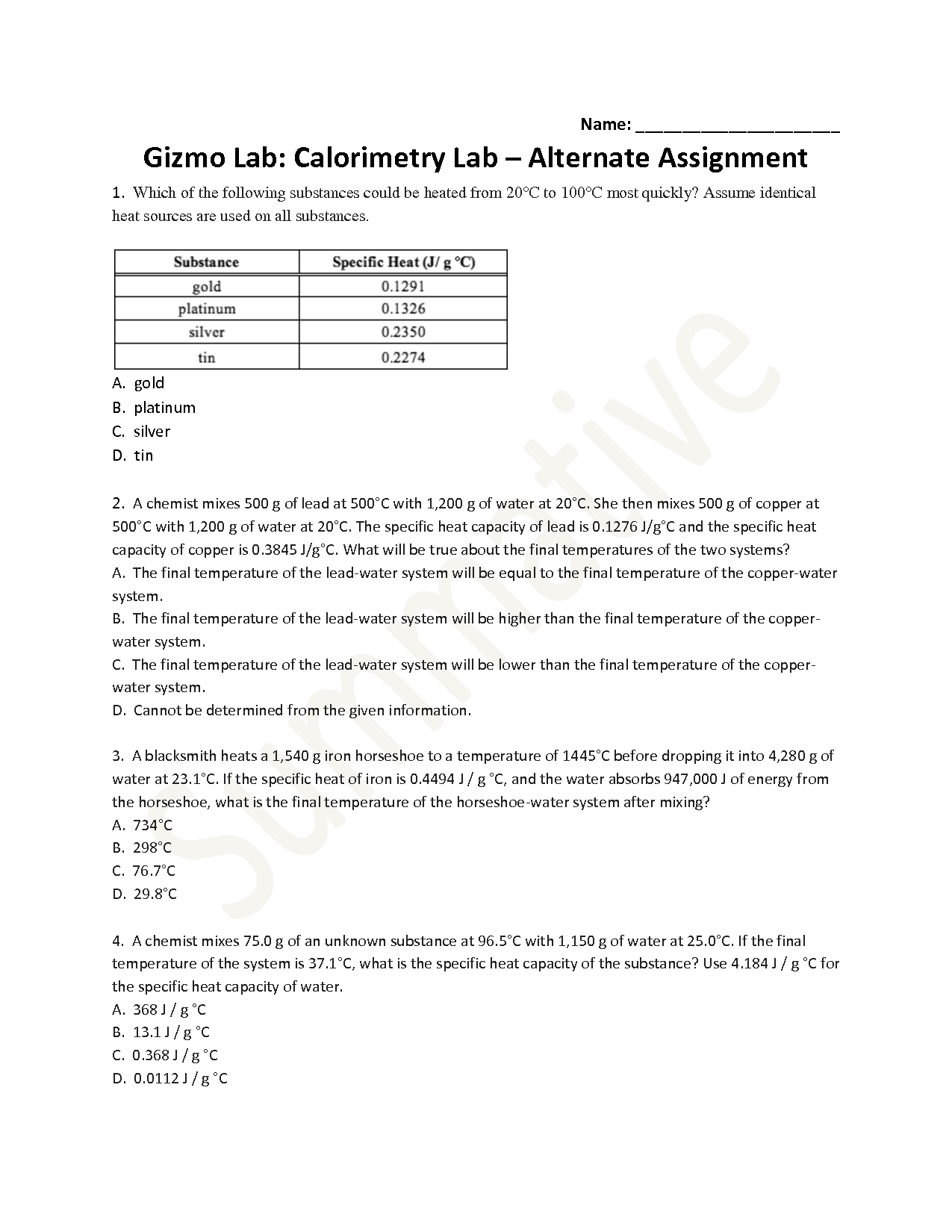
- Student Exploration Chemical Equations Gizmo Answer Key
- For Specific Heat Worksheet Physics
- Calorimetry Practice Worksheet Answers
- Chemistry Specific Heat Worksheet
- Student Exploration Gizmo Photosynthesis Lab Answer Key
- Stoichiometry Worksheet Answer Key
- Celsius of Aluminum Specific Heat
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
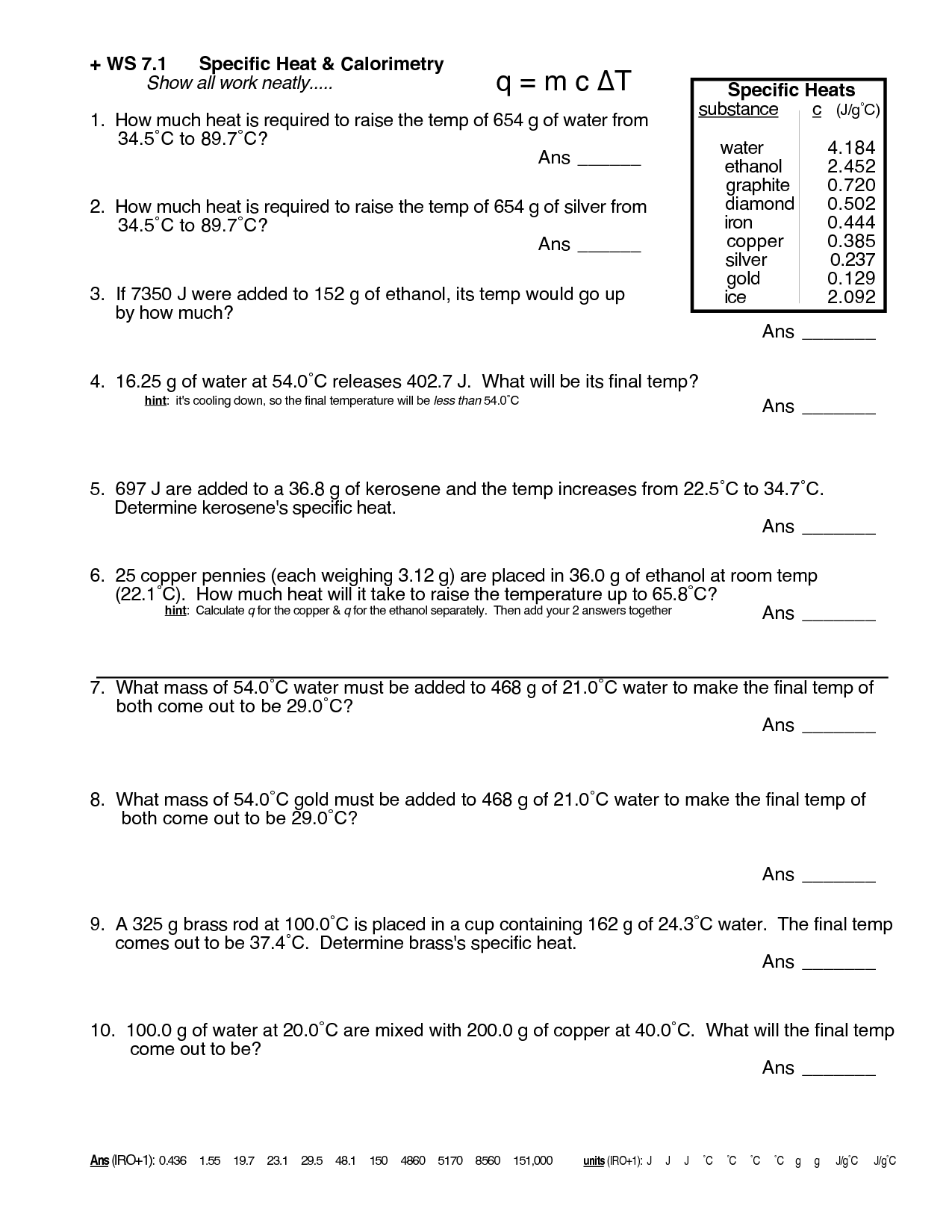
What is specific heat?
Specific heat is the amount of heat energy required to raise the temperature of a specific amount of a substance by one degree Celsius (or Kelvin). It is a physical property that varies for different materials and is important for understanding how substances absorb and release heat.
How is specific heat measured?
Specific heat is measured by determining the amount of heat required to raise the temperature of a known mass of a substance by a certain amount. This is typically done through experiments where the substance is heated or cooled while the amount of heat transferred is measured. The specific heat capacity of a material is then calculated by dividing the amount of heat by the mass and temperature change.
What is the equation for calculating the heat energy absorbed or released?
The equation for calculating the heat energy absorbed or released is Q = mc?T, where Q represents the heat energy, m is the mass of the substance, c is the specific heat capacity of the substance, and ?T is the change in temperature.
How does specific heat differ between different substances?
Specific heat is a measure of how much heat energy a substance can store per unit of mass. Different substances have different specific heat capacities because of variations in their atomic and molecular structures. For example, substances with stronger intermolecular forces, such as water, tend to have higher specific heat capacities because more heat energy is required to break these forces and increase the temperature of the substance. In contrast, substances with weaker intermolecular forces, such as metals, tend to have lower specific heat capacities because they require less heat energy to increase their temperature.
How does specific heat affect the temperature change of a substance?
Specific heat is a measure of the amount of energy required to raise the temperature of a substance by 1 degree Celsius. The higher the specific heat of a substance, the more energy it requires to increase its temperature. Therefore, substances with a higher specific heat will experience a smaller temperature change for a given amount of heat added or removed compared to substances with a lower specific heat. In other words, substances with a higher specific heat will heat up or cool down more slowly than those with a lower specific heat.
What is the specific heat of water?
The specific heat of water is approximately 4.184 joules per gram per degree Celsius (J/g°C).
How does the specific heat of a substance affect its ability to store heat?
The specific heat of a substance directly affects its ability to store heat. A higher specific heat means that the substance can absorb more heat energy before its temperature increases significantly, while a lower specific heat means the substance requires less heat energy to raise its temperature. Therefore, substances with higher specific heats can store more heat energy due to their capacity to absorb and retain more heat without experiencing as large of a temperature change.
How is the specific heat of a substance determined experimentally?
The specific heat of a substance is determined experimentally by measuring the amount of heat required to change the temperature of a known mass of the substance by a certain amount. This is typically done using a calorimeter, a device that can accurately measure heat transfer. By applying a known amount of heat to the substance and measuring the resulting temperature change, the specific heat capacity of the substance can be calculated using the formula Q = mc?T, where Q is the heat added, m is the mass of the substance, c is the specific heat capacity, and ?T is the change in temperature.
How does specific heat affect the efficiency of heating and cooling systems?
Specific heat plays a significant role in the efficiency of heating and cooling systems because it determines the amount of energy required to raise or lower the temperature of a substance. Materials with low specific heat require less energy to heat up or cool down, making them more efficient for heating and cooling systems as they can quickly change temperature. Conversely, materials with high specific heat require more energy, which can lead to decreased efficiency as more energy is needed to reach the desired temperature. Therefore, selecting materials with an appropriate specific heat can help improve the overall efficiency of heating and cooling systems.
Can specific heat be changed or modified?
The specific heat of a substance is an intrinsic property that depends on the material itself, so it cannot be changed or modified. The specific heat is a constant value for a given substance under specific conditions, such as temperature and pressure. However, the specific heat of a mixture or compound can be calculated based on the specific heats of the individual components.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.































Comments