Specific Heat Problems Worksheet
Are you struggling to grasp the concept of specific heat and having a hard time applying it to real-world problems? Look no further than our specific heat problems worksheet! This resource is designed for students who are currently studying thermodynamics and need extra practice calculating specific heat. Whether you're in high school or college, this worksheet will provide you with a variety of problems that will help solidify your understanding of this important concept.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is specific heat?
Specific heat is the amount of heat energy required to raise the temperature of one unit mass of a substance by one degree Celsius (or one Kelvin). It is a characteristic property of a substance and helps determine how much heat energy is needed to change the temperature of the substance. Each substance has its own specific heat value, which is important in various scientific and engineering applications.
How is specific heat measured?
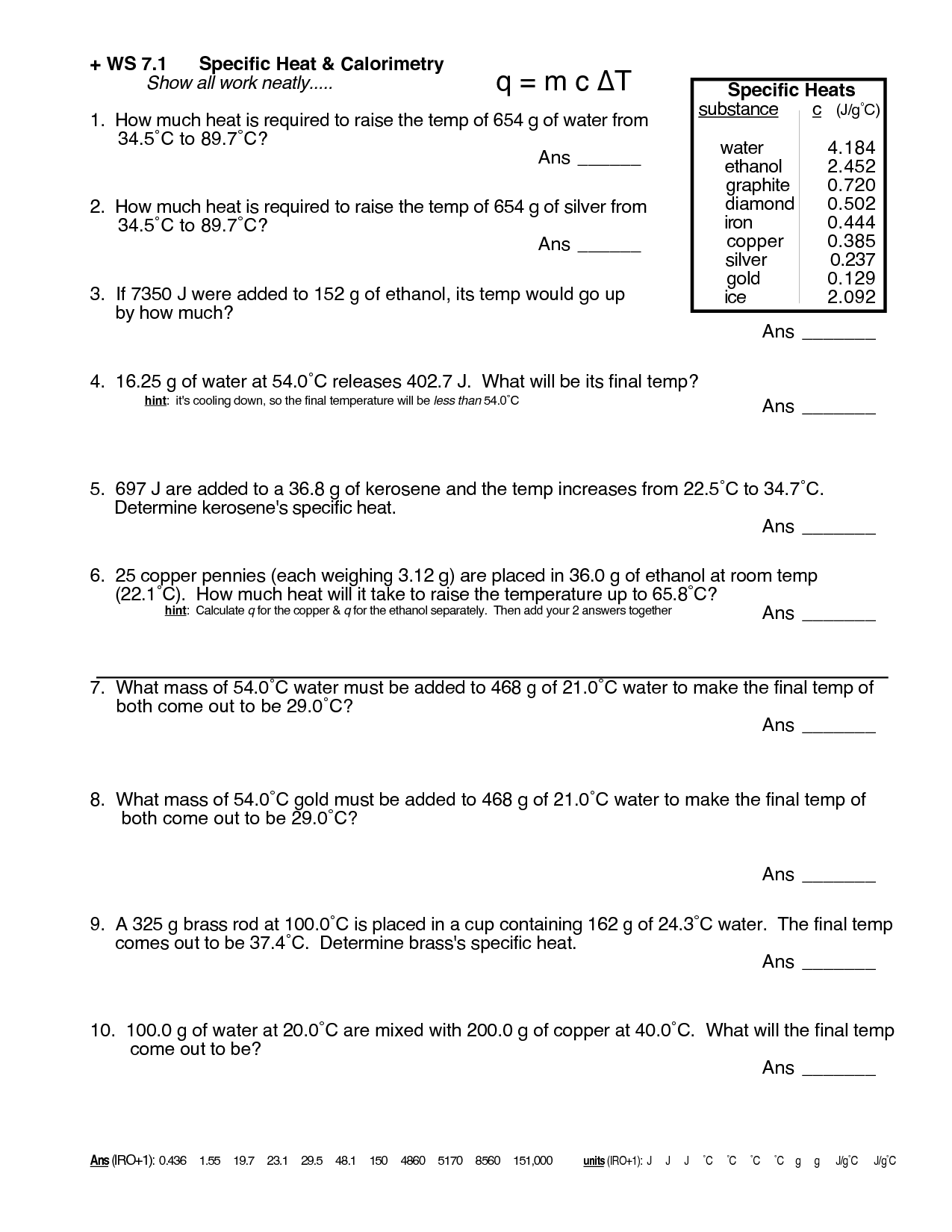
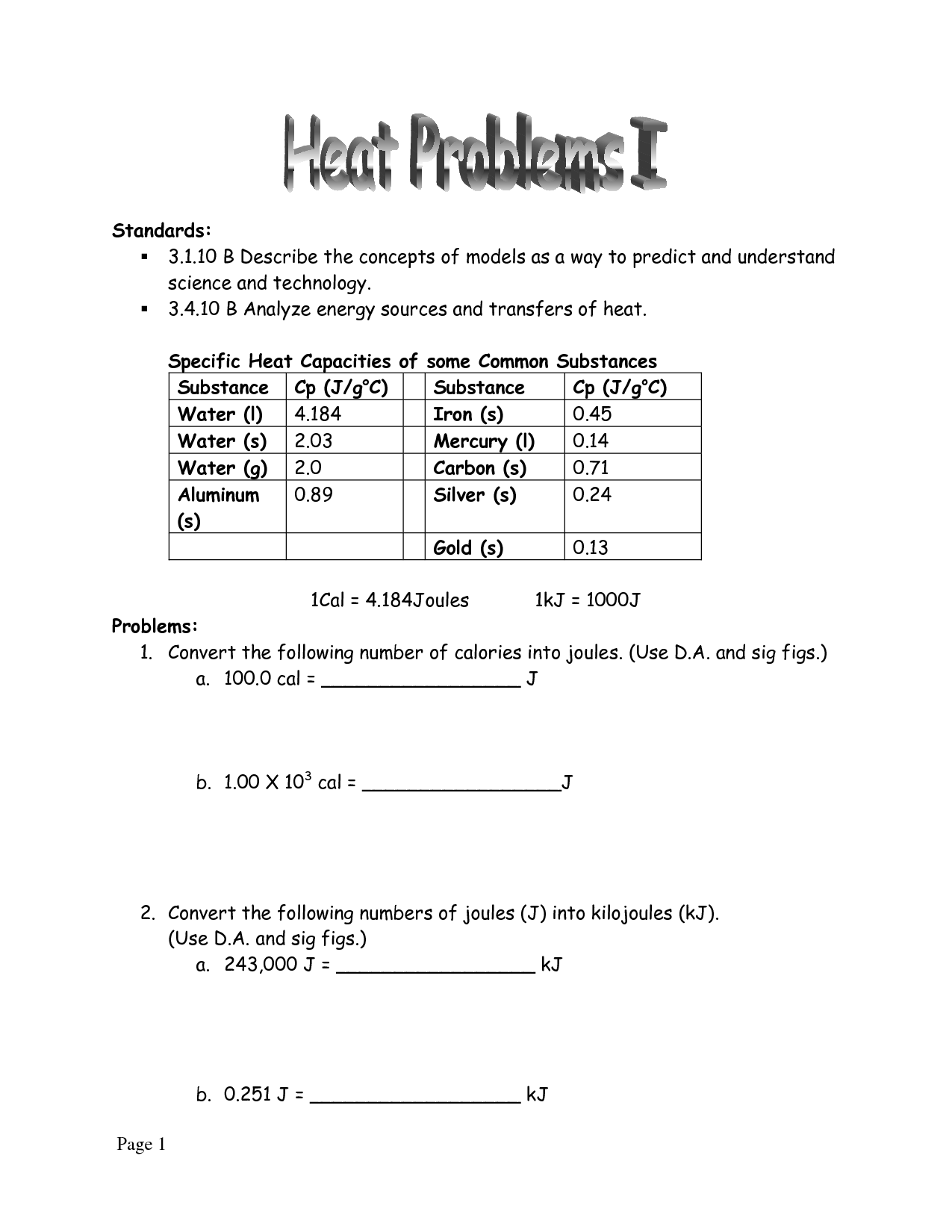
Specific heat is typically measured using a calorimeter, which is a device that accurately determines the amount of heat transferred to or from a substance during a temperature change. The process involves measuring the initial and final temperatures of a sample, determining the amount of heat absorbed or released, and calculating the specific heat capacity using the formula Q=mc?T, where Q is the amount of heat transferred, m is the mass of the sample, c is the specific heat capacity, and ?T is the temperature change.
What is the equation for calculating heat energy?
The equation for calculating heat energy is Q = mc?T, where Q represents the heat energy transferred, m is the mass of the substance, c is the specific heat capacity of the substance, and ?T is the change in temperature of the substance.
How does specific heat affect the temperature change of a substance?
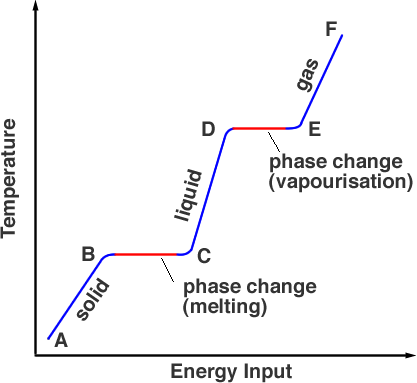
Specific heat is the amount of heat energy required to raise the temperature of a substance by 1 degree Celsius. Substances with a higher specific heat require more heat energy to raise their temperature, causing them to heat up or cool down more slowly compared to substances with lower specific heat. Therefore, the specific heat of a substance affects the temperature change of the substance by determining how much heat energy is needed to cause a change in temperature.
What is the specific heat of water?
The specific heat of water is 4.184 joules per gram per degree Celsius (J/g°C).
How does the specific heat of different substances compare?
The specific heat of different substances varies, with water having a relatively high specific heat compared to most substances. This means that it requires more energy to raise the temperature of water compared to other substances. In contrast, metals like aluminum have lower specific heat values, which means they heat up or cool down faster compared to water. Overall, the specific heat of a substance determines how much heat energy is required to change its temperature, with water being a notable example due to its high specific heat capacity.
How does the mass of a substance affect its specific heat?
The mass of a substance does not affect its specific heat. Specific heat is an intrinsic property of a material that indicates how much heat energy is required to raise the temperature of a unit mass of the substance by one degree Celsius. This property remains constant regardless of the amount of the substance present.
How does the specific heat of a substance affect its ability to store heat energy?
The specific heat of a substance is a measure of how much heat energy it can absorb or release before its temperature changes. A substance with a high specific heat can store more heat energy without experiencing a big change in temperature, while a substance with a low specific heat will experience a greater change in temperature with the same amount of heat energy. Therefore, a substance with a high specific heat has a greater ability to store heat energy compared to a substance with a low specific heat.
How can specific heat be used to solve problems involving temperature changes?
Specific heat can be used to solve problems involving temperature changes by using the formula q = mc?T, where q represents the amount of heat absorbed or released, m is the mass of the substance, c is the specific heat capacity, and ?T is the change in temperature. By rearranging the formula, one can calculate any of these variables given the other information. This helps in determining the amount of heat needed to change the temperature of a substance, or in calculating the temperature change resulting from a given amount of heat input.
What are some real-life applications that involve specific heat?
Specific heat is commonly used in various real-life applications, such as in cooking and baking to determine the required amount of heat energy to cook food evenly, in engineering to calculate the energy needed to heat or cool a building, in the automotive industry to understand how materials respond to temperature changes in engine components, and in meteorology to study the heating and cooling of the atmosphere. Additionally, specific heat is important in industries like pharmaceuticals, for accurately measuring and controlling the temperature during drug manufacturing processes.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.


































Comments