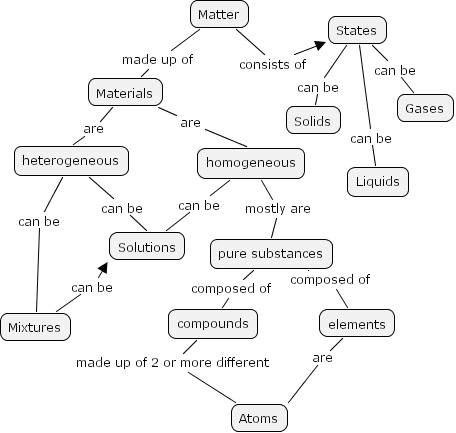
Solids Liquids and Gases Worksheets 8th Grade
Are you an 8th-grade student looking for worksheets to enhance your understanding of solids, liquids, and gases? Look no further! In this blog post, we will introduce you to a variety of worksheets that focus on this fascinating topic. These worksheets will help you strengthen your knowledge and grasp the key concepts related to the properties and behaviors of these three states of matter.
Table of Images 👆
What is the main characteristic of a solid?
The main characteristic of a solid is that it has a definite shape and volume, meaning its particles are closely packed together and vibrate in place, giving the solid a fixed structure.
How do the particles in a solid move?
The particles in a solid vibrate in fixed positions due to strong intermolecular forces. While they do not have the freedom to move around like particles in a liquid or gas, they can still vibrate in place. This vibrating motion is what gives solids their rigidity and shape, as the particles maintain their relative positions even though they are in constant motion.
Give an example of a substance that exists in a liquid state.
Water is a common example of a substance that exists in a liquid state at room temperature.
What happens to the volume and shape of a liquid?
The volume of a liquid remains constant when poured into a container, while the shape of the liquid changes to fit the shape of the container. Liquids take the shape of their container due to their ability to flow and conform to the boundaries of the container they are in, unlike solids which have a fixed shape and volume.
Explain the term "vaporization" in the context of gases.
Vaporization refers to the process by which a substance transitions from a liquid state to a gas or vapor state. This change occurs when the substance's temperature reaches its boiling point and enough energy is added to break the attractive forces between molecules, allowing them to escape and become a gas. The main types of vaporization are evaporation, which is a surface phenomenon where molecules escape directly from a liquid's surface, and boiling, which occurs throughout the liquid as bubbles of vapor form.
Provide an example of a gas in everyday life.
An example of a gas in everyday life is the oxygen that we breathe. It is a colorless, odorless gas that makes up about 21% of the Earth's atmosphere and is essential for human and animal life.
What is the key characteristic of gases in terms of their shape and volume?
The key characteristic of gases is that they have neither a definite shape nor a definite volume. Gases are able to fill the entire space of their container, taking on the shape of the container they are in, and their volume can easily change in response to external conditions like pressure and temperature.
Describe the process of condensation in the context of liquids.
Condensation is the process in which a substance transitions from its gaseous state to its liquid state. This occurs when the temperature of the gas decreases, causing the particles to slow down and come closer together, thereby forming liquid droplets. The reverse of evaporation, condensation is a phase change phenomenon often observed when warm, moist air comes into contact with a cooler surface, leading to the formation of dew or fog.
How are the particles arranged in a solid compared to a liquid or gas?
In a solid, particles are tightly packed together in an orderly arrangement, forming a fixed shape and volume. In contrast, in a liquid, particles are still close together but can move past each other, resulting in a fixed volume but an indefinite shape. In a gas, particles are far apart and move freely, leading to both an indefinite shape and volume.
Explain the concept of melting in relation to solids becoming liquids.
Melting is the process through which a solid substance changes into a liquid state when heat is applied. As thermal energy is added to the solid, the particles in the solid start vibrating more rapidly, eventually overcoming the forces holding them in a rigid, fixed position. This increased kinetic energy causes the particles to break free from their fixed positions and move more freely, transitioning the substance from a solid to a liquid state.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.















Comments