Solid and Liquid Matching Worksheets
The solid and liquid matching worksheets are designed to help students in the elementary grades understand the differences between solids and liquids. These worksheets provide an engaging and interactive way for young learners to identify and classify various objects according to their physical properties. By engaging with these exercises, students will develop a clearer understanding of the characteristics of solids and liquids and enhance their scientific knowledge.
Table of Images 👆
- Printable Matter Worksheets
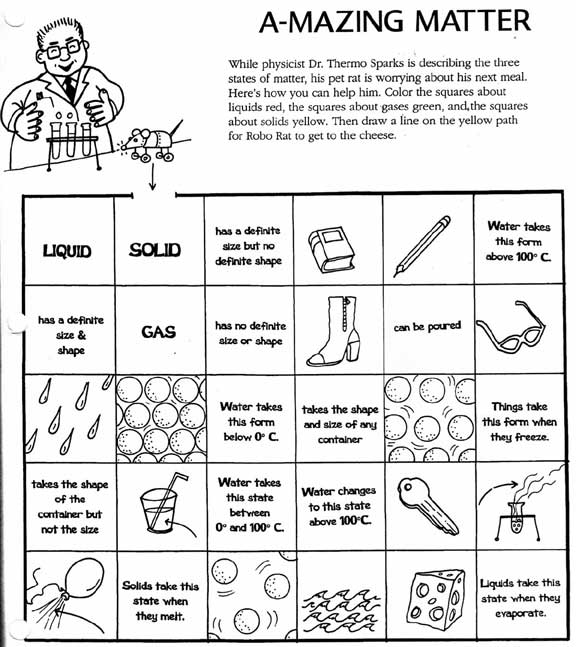
- Solid-Liquid Gas Worksheet
- Matter Solid-Liquid Gas Worksheet
- Solid Liquid and Gas Worksheets
- States of Matter Worksheets Grade 2
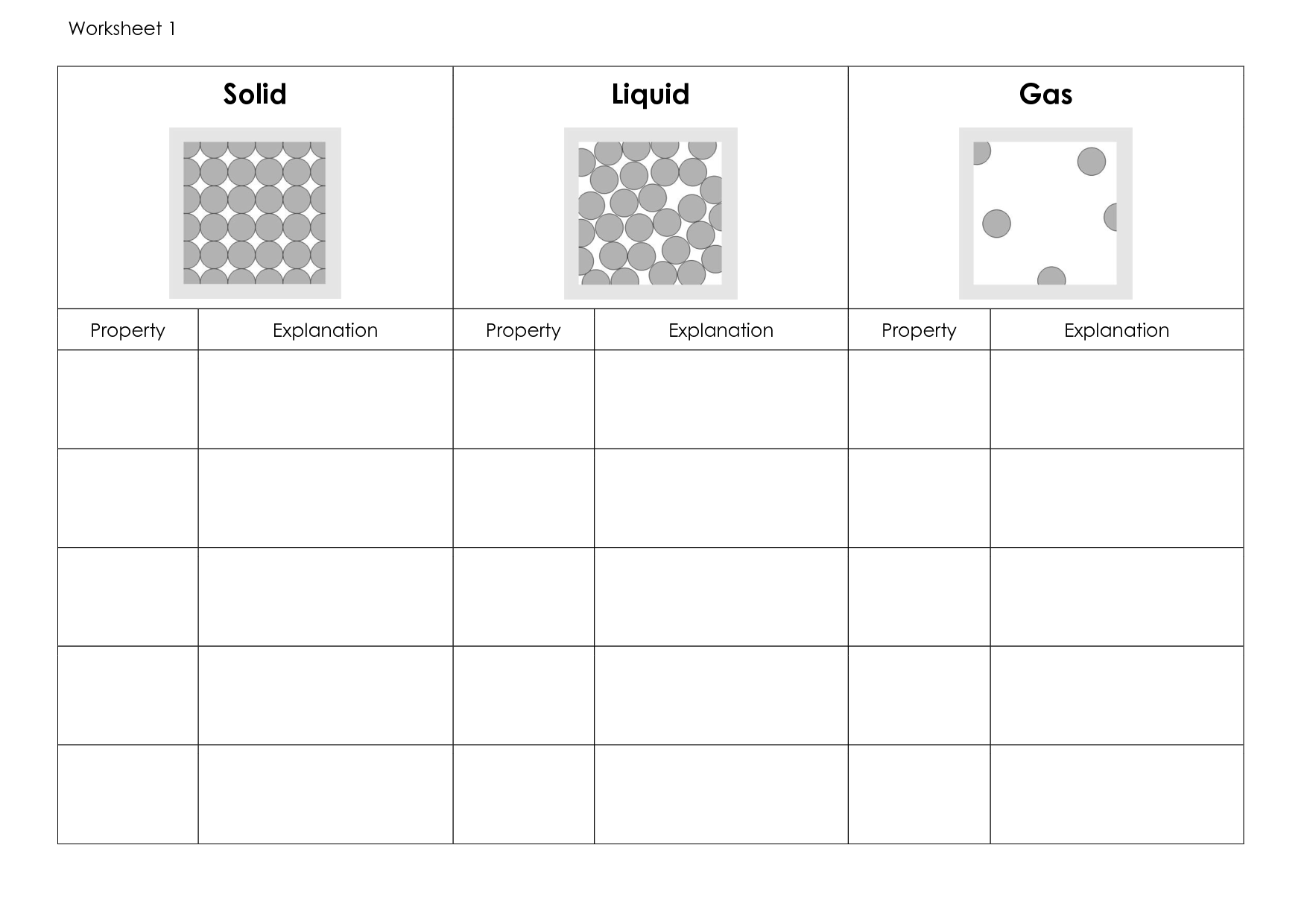
- State of Matter Gas Liquid and Solids Worksheets
- States of Matter Worksheets Middle School
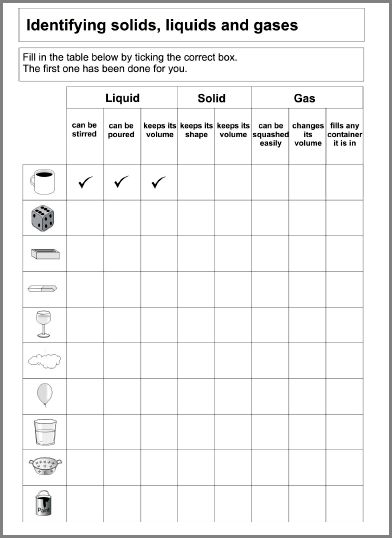
- Solids Liquids and Gases Worksheets
- 2nd Grade Solids Liquids and Gases Worksheets
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
How does the shape of a solid differ from that of a liquid?
The shape of a solid remains fixed and maintains a definite volume, whereas a liquid takes the shape of its container and does not have a fixed shape. Solids have a definite structure and particles that are closely packed together, maintaining their shape under normal conditions. Liquids, on the other hand, have particles that are not tightly packed, allowing them to flow and take the shape of their container.
What happens to the molecular arrangement in a solid compared to a liquid?
In a solid, the molecules are tightly packed together in a fixed arrangement and vibrate in place. However, in a liquid, the molecules have more energy and are able to move past each other, resulting in a less ordered arrangement compared to a solid.
What are the intermolecular forces like in a solid?
In a solid, the intermolecular forces are typically strong, resulting in closely packed and ordered arrangements of molecules or atoms. These forces can include ionic, covalent, metallic, or van der Waals interactions, depending on the specific solid material. The strength of these intermolecular forces contributes to the high stability and rigidity of solids, as they hold the particles in fixed positions relative to each other.
How do the particles in a liquid move compared to those in a solid?
In a liquid, particles are more free to move compared to those in a solid. They have more energy and are able to slide past each other, allowing liquids to flow and take the shape of their container. In contrast, particles in a solid are more tightly packed and have less energy, vibrating in place without the freedom to move around as freely as in a liquid.
How do the properties of a solid differ from those of a liquid?
Solids have a fixed shape and volume, with particles packed closely together in a regular pattern, giving them a definite structure. Liquids, on the other hand, take the shape of their container and have a fixed volume, with particles that are close together but can move past each other, giving liquids the ability to flow. Additionally, solids have higher densities compared to liquids, and solids typically have higher melting points than liquids.
What changes occur in the volume and shape of a solid when heated?
When a solid is heated, its volume typically expands as the particles within the solid gain energy and move more freely. This expansion causes the solid to increase in size. Additionally, the shape of the solid may also change if it reaches its melting point and transitions into a liquid state.
How does the density of a liquid compare to that of a solid?
The density of a liquid is typically lower than that of a solid because the molecules in a liquid are able to move more freely and are less densely packed compared to those in a solid. This is why liquids typically have a lower density and are able to flow and take the shape of their container, while solids have a fixed shape and volume due to their closely packed molecules.
Can liquids be compressed like solids?
No, liquids cannot be compressed like solids because they do not have a definite shape or volume. Unlike solids, which have tightly packed molecules in a fixed arrangement, the molecules in liquids are not arranged in a specific order and can move around freely, making them difficult to compress.
What happens to the surface tension of a liquid when it is heated?
When a liquid is heated, its surface tension typically decreases. This is because increased temperature causes the molecules at the surface of the liquid to have higher kinetic energy, allowing them to overcome the cohesive forces that create surface tension. As a result, the molecules at the surface become more mobile and are less likely to stick together, leading to a reduction in surface tension.
How do the viscosity and flow of a liquid change with temperature compared to a solid?
The viscosity of a liquid typically decreases with increasing temperature due to the increase in molecular movement and decreased intermolecular forces. This results in a smoother flow of the liquid. In contrast, the flow of a solid is relatively unaffected by temperature due to its rigid and fixed molecular structure, maintaining a consistent flow behavior regardless of temperature changes.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.




























Comments