Separating Mixtures Worksheet for 5th
This Separating Mixtures worksheet is designed specifically for 5th grade students who are learning about the different methods of separating mixtures. Whether you're a teacher looking for supplemental materials or a parent seeking extra practice for your child, this worksheet provides a comprehensive review of the topic.
Table of Images 👆
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
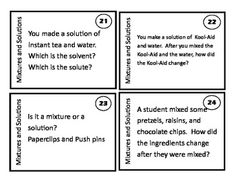
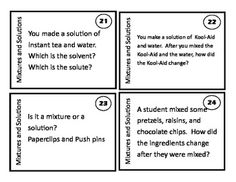
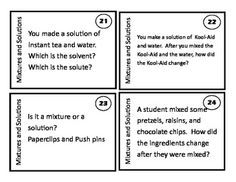
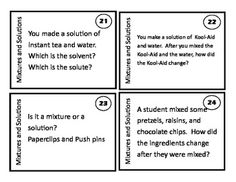
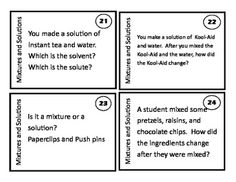
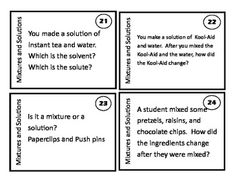
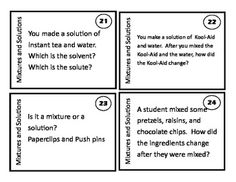
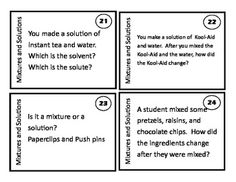
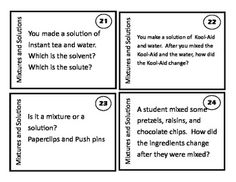
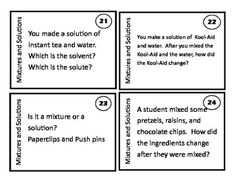
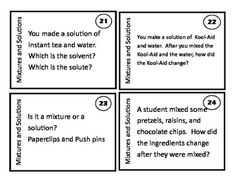
What is a mixture?
A mixture is a combination of two or more substances that are physically combined but not chemically bonded together. Each substance retains its own properties in a mixture, and they can be separated through physical means like filtration or distillation.
Explain the difference between a homogeneous mixture and a heterogeneous mixture.
A homogeneous mixture has uniform composition and properties throughout, with its components being evenly distributed at a molecular level, such as saltwater or air. In contrast, a heterogeneous mixture contains visibly different substances that are not uniformly distributed, like a salad or a mixture of oil and water.
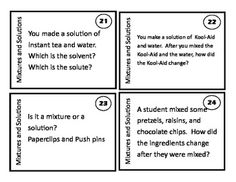
What is filtration and how is it used to separate mixtures?
Filtration is a process used to separate solids from liquids or gases by passing the mixture through a porous substance, such as filter paper or a filter funnel. As the mixture flows through the filter, the larger particles or solids are trapped while the liquid or gas passes through. This method allows for the separation of components based on differences in particle size, density, or solubility, making it a useful technique for purifying substances and isolating specific components from a mixture.
Describe the process of distillation and its purpose in separating mixtures.
Distillation is a process that involves heating a mixture to separate its components based on differences in their boiling points. The mixture is heated to vaporize the more volatile component, which is then collected and condensed back into a liquid form. The purpose of distillation is to separate and purify the components of a mixture, especially when they have significantly different boiling points, enabling the extraction of a desired substance from a complex mixture. By utilizing the principle of boiling point differences, distillation allows for the isolation of individual components, making it a valuable technique in various industries such as pharmaceuticals, food and beverage, and chemical production.
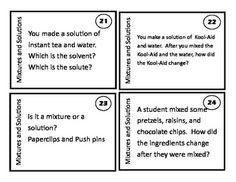
How does evaporation separate a mixture? Provide an example.
Evaporation separates a mixture by utilizing the different boiling points of the components. For example, in a mixture of saltwater, when the solution is heated, the water evaporates, leaving behind the salt. This is possible because water has a lower boiling point than salt, allowing the water to evaporate first, leaving the salt behind in the original container.
What is chromatography and how does it separate mixtures?
Chromatography is a technique used to separate and analyze mixtures of substances based on their different affinities for a stationary phase and a mobile phase. The stationary phase is typically a solid or liquid material, while the mobile phase is a liquid or gas that carries the sample through the stationary phase. As the sample travels through the stationary phase, the components of the mixture interact differently with the stationary and mobile phases, causing them to separate based on their differing chemical properties such as size, charge, or polarity. This results in the components moving at different rates and thus being separated for identification or further analysis.
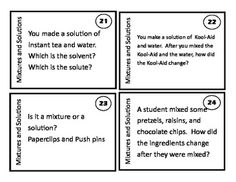
Explain the technique of magnetism in separating mixtures.
Magnetism is a technique used to separate mixtures containing magnetic materials. By applying a magnetic force to the mixture, the magnetic components are attracted to the magnet and can be easily separated from the non-magnetic components. This method is commonly used in industries such as mining to separate iron ore from other materials. It is a simple and efficient technique for separating mixtures based on their magnetic properties.
How does solubility help to separate mixtures? Give an example.
Solubility plays a key role in separating mixtures through a process called liquid-liquid extraction. In this technique, the components of a mixture are dissolved in different solvents based on their solubility properties. For example, if a mixture contains both water-soluble and non-water-soluble components, adding a solvent that selectively dissolves one component while leaving the other insoluble allows for the separation of the two components by physically separating the layers of the solvent mixture.
Describe the process of sieving and its use in separating mixtures.
Sieving is a method of separating particles based on their size. It involves passing a mixture through a sieve, which is a device with evenly spaced holes of different sizes. The larger particles are retained on top of the sieve, while the smaller particles pass through. This process is commonly used to separate materials such as sand and stones from a mixture, allowing for the removal of unwanted particles based on their size. Sieving is a simple and effective method for separating mixtures based on particle size differences.
How can density be used to separate mixtures?
Density can be used to separate mixtures by exploiting the differences in density between the components of the mixture. By adding a liquid or medium with a specific density to the mixture, the components will separate based on their density. The lighter components will float on top while the heavier ones will sink to the bottom, allowing for easy separation. This process is commonly used in techniques such as centrifugation or filtration to isolate and purify substances in a mixture based on their density differences.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.
























Comments