Properties of Matter Worksheet
Are you a science teacher in search of a comprehensive and engaging resource for teaching about the properties of matter? Look no further than our Properties of Matter Worksheet. This informative and interactive worksheet is designed to help your students explore various concepts related to matter and its properties. With carefully crafted questions targeting different aspects of matter, this worksheet is perfect for middle school students who are eager to enhance their understanding of this fundamental topic.
Table of Images 👆
- Physical vs Chemical Properties Worksheet
- Physical Property of Matter Worksheet
- Physical Properties and Chemical Changes Worksheet
- States of Matter Worksheets 5th Grade
- Physical and Chemical Properties Worksheet
- List Return to Matter Worksheets
- Properties of Matter Unit
- Properties of States of Matter Worksheet
- Physical Matter Properties Worksheet
- Properties of Matter Activities
- Physical Properties of Matter Worksheet
- Properties of Matter Worksheet Answers
- Pure Substances and Mixtures Worksheet
- Physical and Chemical Property of Matter Worksheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
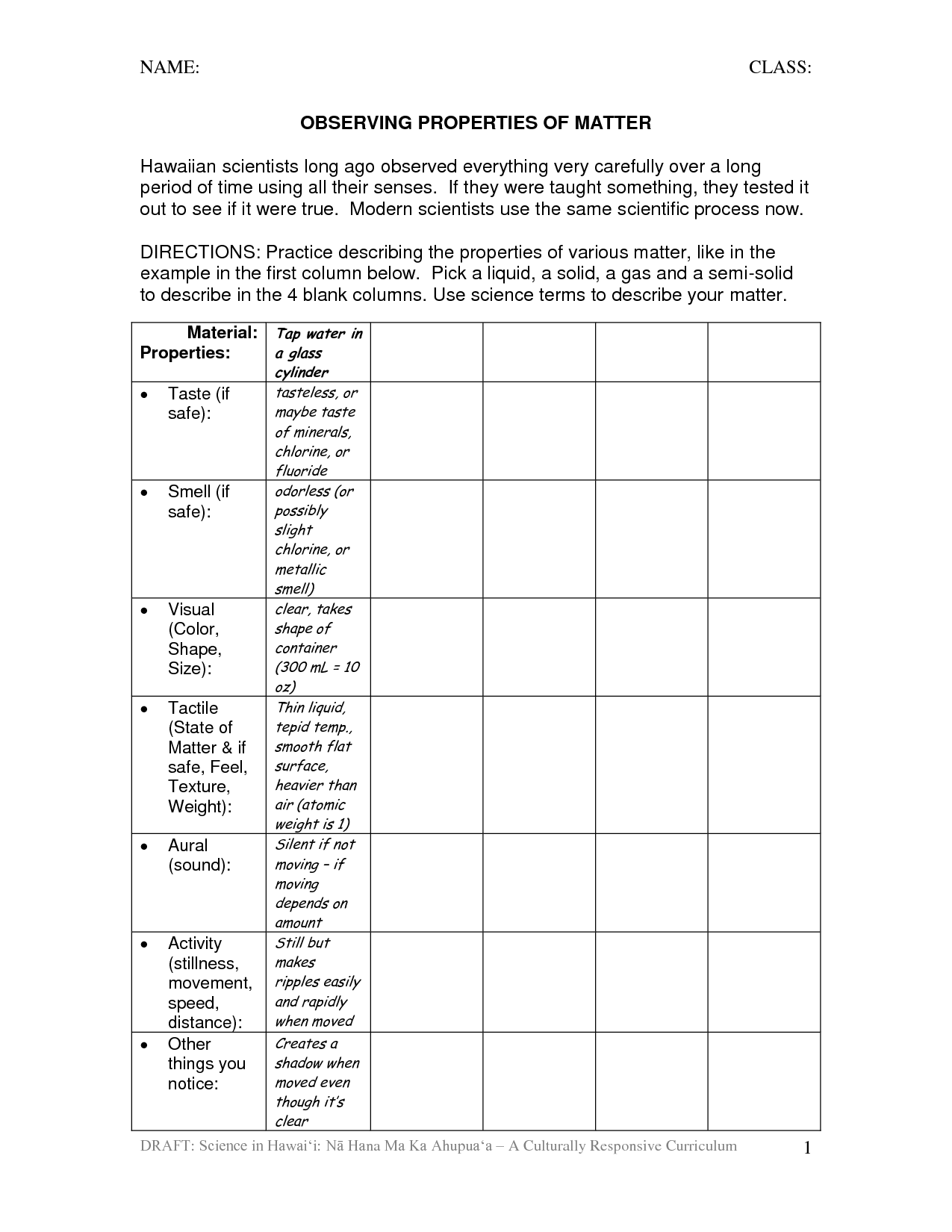
What is the definition of matter?
Matter is defined as anything that has mass and takes up space; it consists of particles such as atoms and molecules.
What are the three states of matter?
The three states of matter are solid, liquid, and gas.
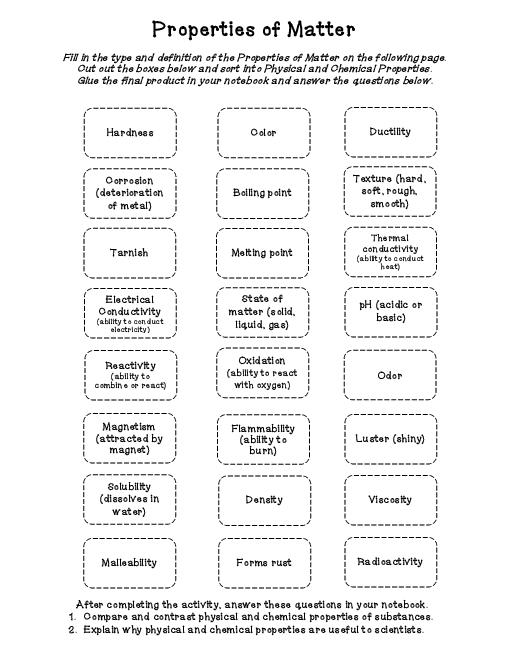
What is the difference between a physical and a chemical property?
A physical property is a characteristic of a substance that can be observed or measured without changing the composition of the substance, such as color, shape, density, or melting point. On the other hand, chemical properties describe how a substance interacts with other substances and undergoes chemical reactions, including its reactivity, flammability, and ability to corrode. Essentially, physical properties are observable attributes, while chemical properties involve the behavior of a substance in reactions.
Give an example of a physical property of matter.
Density is an example of a physical property of matter. Density is the mass of a substance per unit volume, and is an intrinsic property that remains constant regardless of the amount of substance present. It is used to characterize and identify different materials based on their mass-to-volume ratio.
What is density and how is it calculated?
Density refers to the measure of how much mass is contained in a given volume of a substance. It can be calculated by dividing the mass of an object by its volume. The formula for density is: Density = Mass/Volume. The unit of density is typically measured in grams per cubic centimeter (g/cm^3) or kilograms per cubic meter (kg/m^3).
Describe the process of a physical change.
A physical change is a change in the state or appearance of a substance that does not result in a change in its chemical composition. This process involves altering the physical properties of a substance, such as its state of matter (solid, liquid, gas), size, shape, or texture without forming any new substances. Examples of physical changes include melting, freezing, boiling, condensing, dissolving, crushing, or breaking, where the substance retains its original composition and can often be reversed.
Give an example of a chemical property of matter.
One example of a chemical property of matter is flammability, which is the ability of a substance to burn or ignite when exposed to a flame or heat source. This property helps to determine how a substance may react in particular situations or environments, making it important for safety and handling considerations.
Explain the concept of solubility.
Solubility is the ability of a substance to dissolve in a solvent, typically water, to form a homogeneous mixture. It is influenced by factors such as temperature, pressure, and the chemical nature of the solute and solvent. Substances that readily dissolve in a solvent are considered soluble, while those that do not dissolve are insoluble. The maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature is known as the solubility limit. Solubility is an important property in various chemical processes, as it determines the extent to which a substance can be dissolved and transported in a solution.
Define the terms melting point and boiling point.
The melting point is the temperature at which a solid substance changes into a liquid state, while the boiling point is the temperature at which a liquid substance changes into a vapor or gas state. Both melting and boiling points are characteristic physical properties of a substance and can be used to identify and classify materials.
How can the properties of matter be used to identify substances?
The properties of matter, such as mass, volume, density, melting point, boiling point, solubility, conductivity, and reactivity, can be used to identify substances. By comparing these properties to known values in reference tables or databases, scientists can determine the specific characteristics of a substance. This information can then be used to identify and classify the substance based on its unique set of properties, enabling scientists to differentiate between different substances and understand their chemical composition and behavior.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.






























Comments