Physical Properties of Water Worksheet
Water is a fundamental aspect of our daily lives, and understanding its physical properties is crucial for a wide range of individuals, from students studying science to homeowners wanting to improve their water usage. To delve deeper into the fascinating world of water, a carefully designed worksheet can provide a structured and comprehensive approach, allowing learners to explore the entity and subject of water's physical properties.
Table of Images 👆
- Physical Matter Properties Worksheet
- Properties of Matter Worksheet Answers
- Properties of Logarithms Worksheet Answers
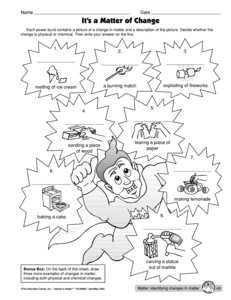
- Physical Chemical Change Worksheets
- Kids Physical and Chemical Changes Worksheet
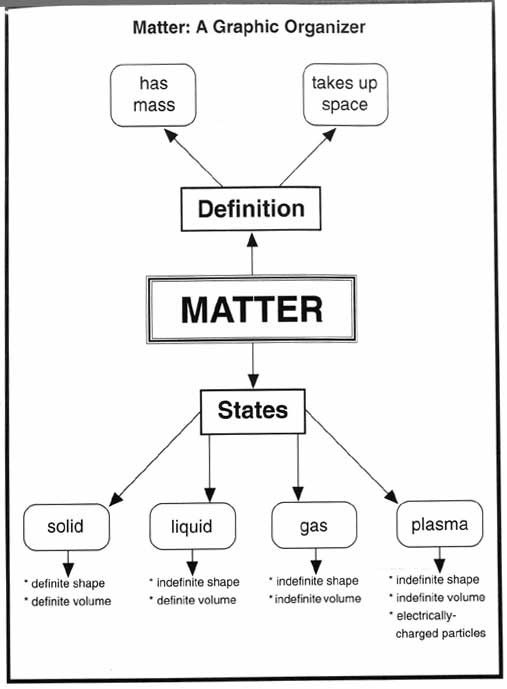
- Matter Science Graphic Organizers
- Physical vs Chemical Change Worksheet
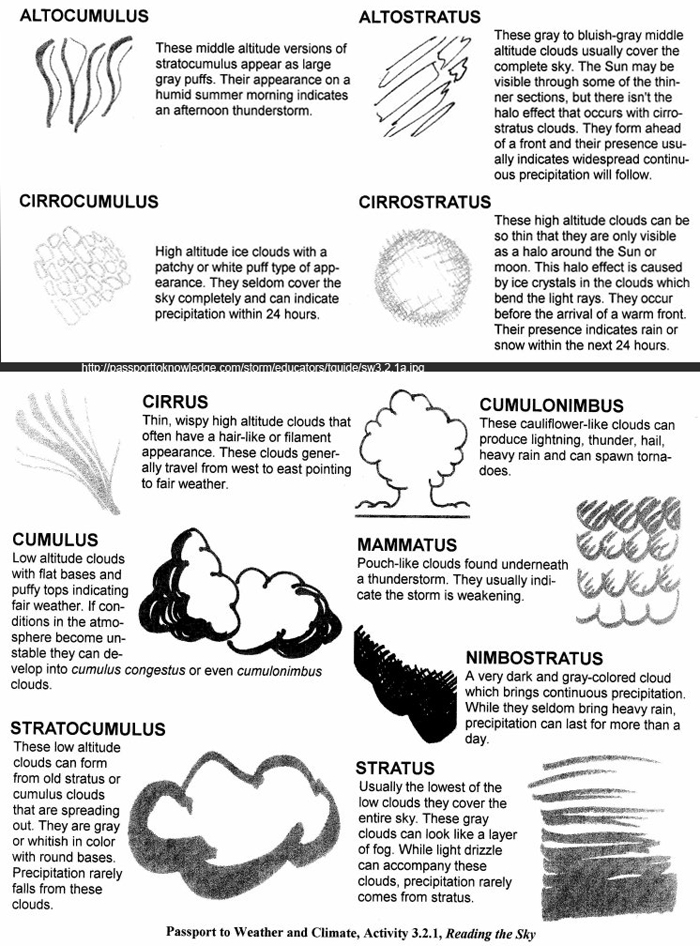
- 5th Grade Science Weather Worksheets
- Element Word Search Answer Key
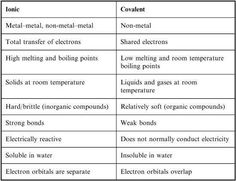
- Ionic and Covalent Bond Chart
- Living and Non-Living Things Worksheets
- Periodic Table with Element Mass
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is the physical state of water at room temperature?
At room temperature, which is typically around 20-25 degrees Celsius (68-77 degrees Fahrenheit), water exists in a liquid state.
How does water behave when it freezes?
When water freezes, its molecules slow down and come closer together, forming a solid crystalline structure. This causes the water to expand and increase in volume, leading to the formation of ice. As a result, ice is less dense than liquid water, which is why ice floats on water. Additionally, the freezing process traps impurities and air bubbles within the ice, creating a translucent appearance.
What is the boiling point of water?
The boiling point of water is 100 degrees Celsius (212 degrees Fahrenheit) at standard atmospheric pressure.
Does water have a high or low viscosity?
Water has a low viscosity, as its molecules can flow easily past each other.
What is the density of water compared to most liquids?
The density of water is higher compared to most liquids, as water has a density of approximately 1 gram per cubic centimeter at standard conditions, making it denser than many other commonly encountered liquids.
Can water dissolve many different substances?
Yes, water is known as the "universal solvent" because it has the ability to dissolve many different substances due to its polar nature. Water molecules are polar, meaning they have a slightly positive and negative end, which allows them to attract and surround other molecules and ions, breaking them apart and forming homogeneous solutions.
Does water have a high or low surface tension?
Water has a relatively high surface tension compared to other liquids, making it difficult to break or penetrate the surface of water due to the cohesive forces between water molecules.
How does water behave when heated?
When water is heated, its molecules gain energy and move faster, causing them to break the hydrogen bonds that hold them together. As a result, the water molecules move apart, leading to an increase in volume and a decrease in density. Eventually, the water molecules reach a point where they have enough energy to escape the liquid phase and turn into water vapor through the process of evaporation.
Does water expand or contract when it freezes?
Water expands when it freezes. This is because the water molecules form a crystalline structure in the solid state, causing them to be farther apart compared to the more densely packed arrangement in liquid form.
How does water's high heat capacity contribute to its physical properties?
Water's high heat capacity, which is the amount of heat energy required to raise its temperature, contributes to several of its physical properties. One significant aspect is water's ability to regulate temperature changes within living organisms and in the environment, making it a stable environment for the survival of aquatic life. Additionally, water's high heat capacity allows it to absorb and release large amounts of heat before experiencing significant temperature changes, which plays a crucial role in moderating Earth's climate and weather patterns. This property also leads to the creation of ocean currents and influences the overall climate and weather patterns on a global scale.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.

























Comments