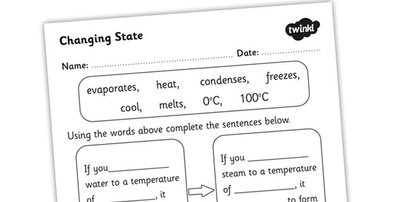
Phase Change Worksheet
Are you a teacher or parent in need of educational resources to support your students or children? Introducing our Phase Change Worksheet, designed to engage and reinforce understanding of entity and subject for students aged 10-12.
Table of Images 👆
- Phase Change Worksheet Answers
- Label Phase Change Diagram
- Phase Diagram Worksheet Answer Key
- Phase Change Worksheet Answer Key
- Phase Change Graph Worksheet Answers
- Kinetic Molecular Theory Worksheet Answers
- Chemistry Phase Diagram Worksheet Answer Key
- Physical and Chemical Properties Worksheet
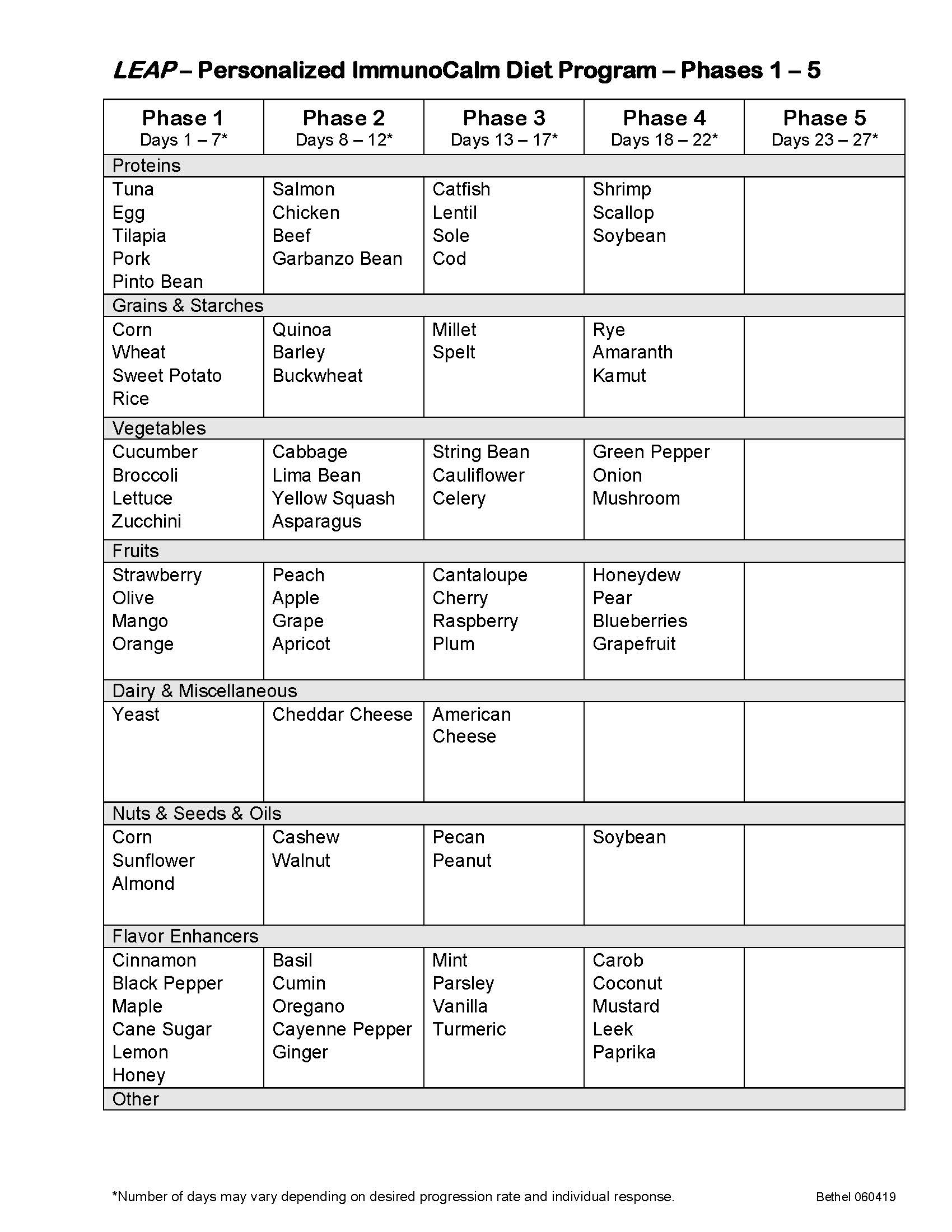
- 13 Day Diet Military Plan
- State Change of Water Worksheet
- Worksheet Mole Problems Answers
- States of Matter Changes Worksheet
- Moon and Stars
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
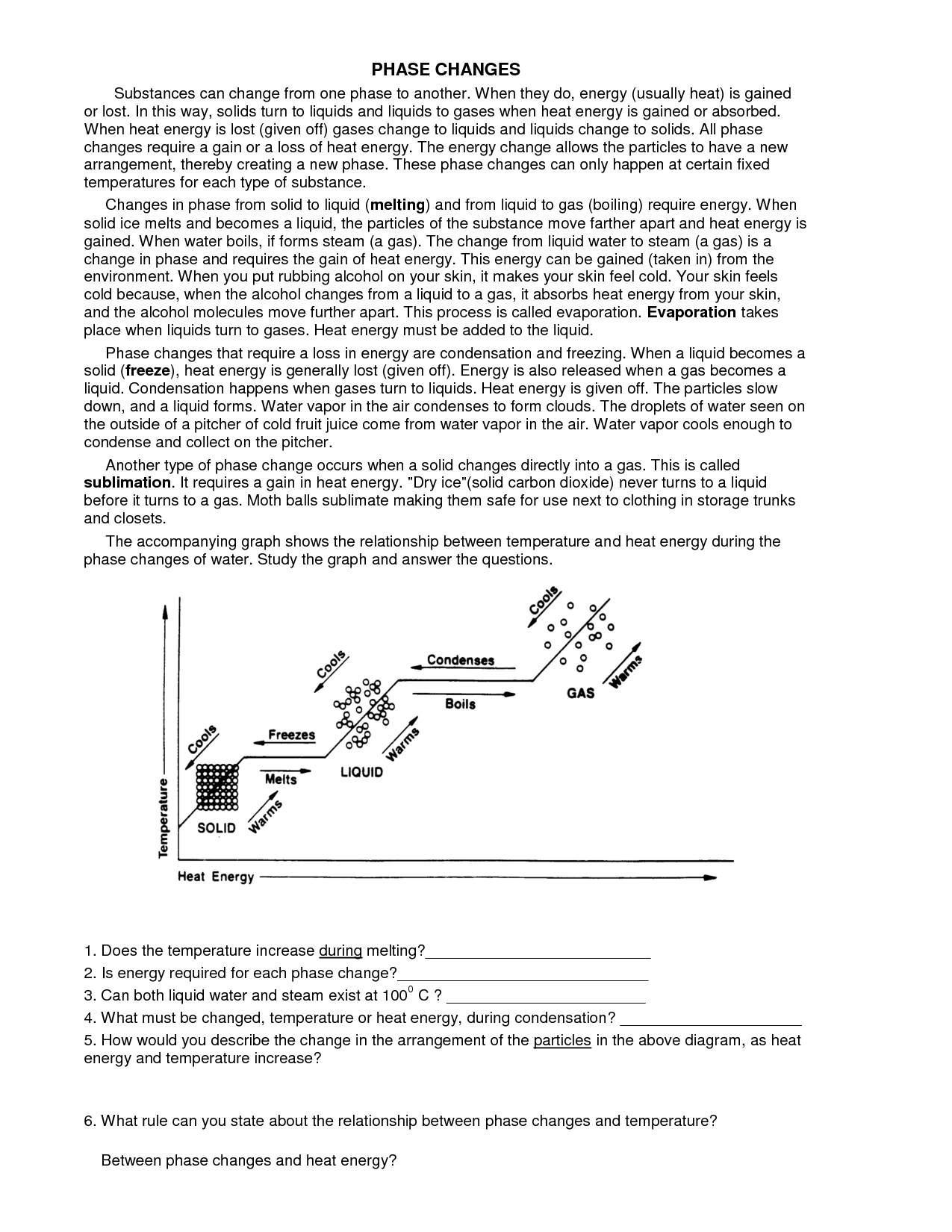
What is meant by a phase change?
A phase change refers to the physical transformation of a substance from one state of matter to another, such as from a solid to a liquid or from a liquid to a gas. During a phase change, the temperature of the substance remains constant as energy is absorbed or released to break or form intermolecular bonds. Each phase change is characterized by a specific heat of transformation and occurs at a distinct temperature under specific conditions.
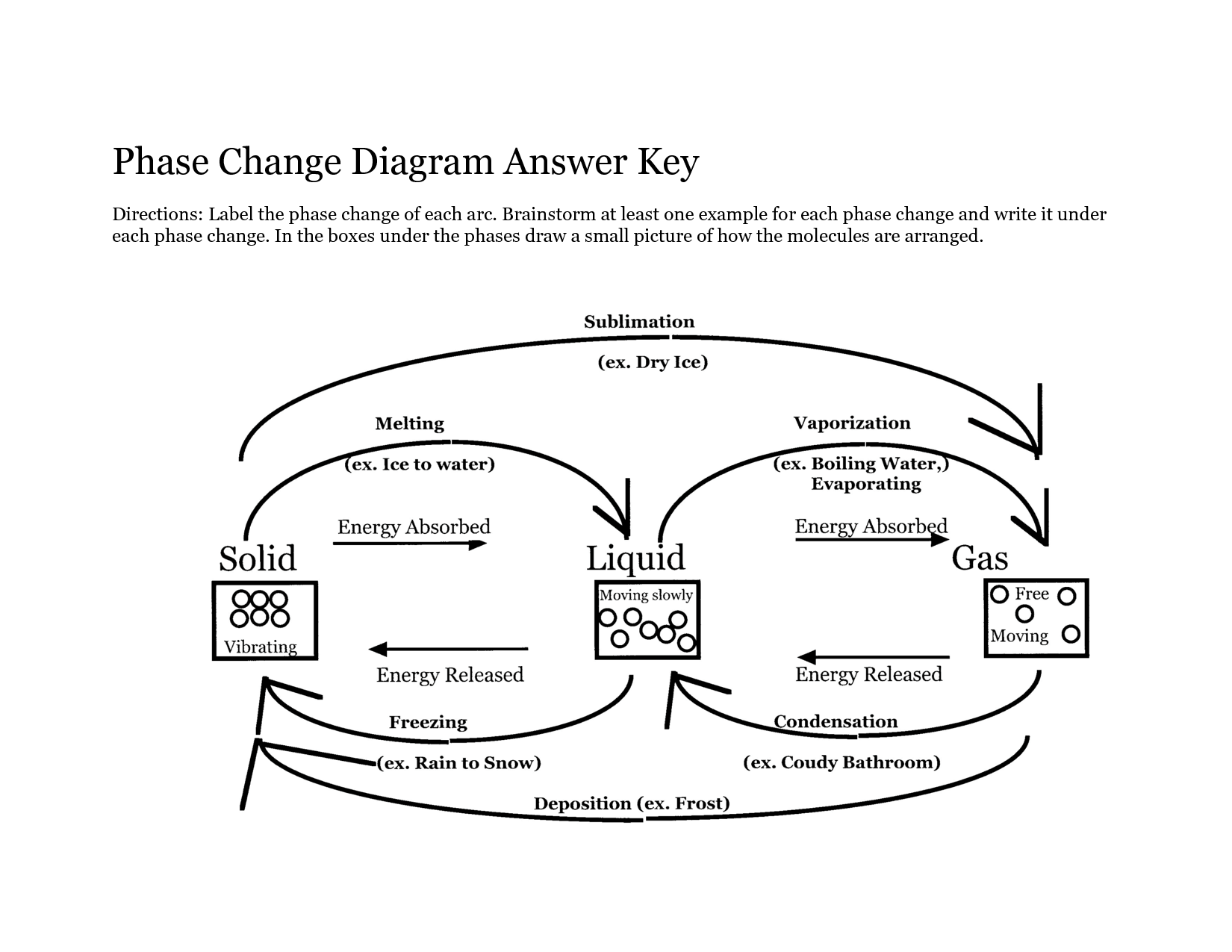
What is the difference between melting and freezing?
Melting is the process in which a solid substance changes into a liquid state by absorbing heat energy, while freezing is the opposite process in which a liquid substance changes into a solid state by releasing heat energy. Melting occurs at the melting point temperature of a substance, where its solid structure breaks down, whereas freezing occurs at the freezing point temperature, where molecules in a liquid slow down and form a solid structure.
Explain the process of evaporation.
Evaporation is the process by which a liquid turns into a gas or vapor due to an increase in temperature. As the temperature of a liquid rises, the molecules gain kinetic energy and start moving more quickly. Some of these fast-moving molecules near the surface of the liquid break free from the liquid's surface tension and escape into the surrounding air as gas. This process continues until the liquid eventually evaporates completely, leaving behind only the solid components if any. Evaporation is a crucial part of the water cycle and plays a key role in the movement of water from the surface of the Earth into the atmosphere.
How does condensation occur?
Condensation occurs when water vapor in the air cools down and loses energy, causing it to change from a gaseous state to a liquid state. This process commonly happens when warm, moist air comes into contact with a colder surface, such as a window or mirror, leading to the formation of water droplets or fog.
Describe the process of sublimation.
Sublimation is a process where a solid substance transforms directly into a gas without passing through the liquid state. This occurs when the atmospheric pressure is lower than the substance's vapor pressure at a specific temperature, allowing the solid to change into gas molecules. As the substance absorbs heat energy, its particles gain enough kinetic energy to break free from the solid structure and disperse as a gas. Sublimation is commonly observed in dry ice (solid carbon dioxide) and certain types of snow where the conditions facilitate this direct transition from solid to gas.
What happens during deposition?
During a deposition, a witness is required to answer questions under oath in a formal setting outside of court. Attorneys from both sides of a legal case are present to ask the witness questions, and a stenographer records a transcript of the proceedings. Depositions are used to gather information, establish witness testimony, and gather evidence for use in a trial or settlement negotiations.
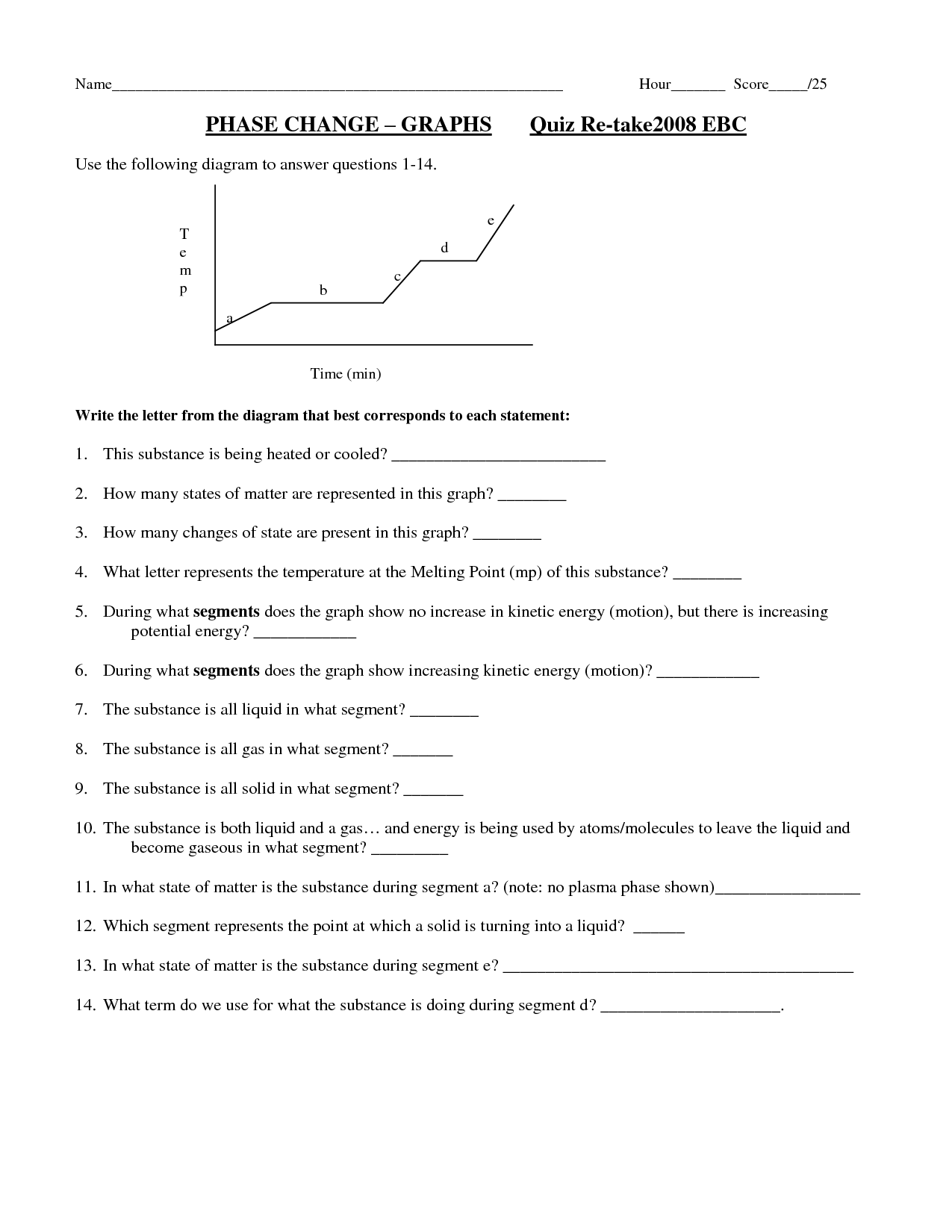
What is the role of temperature in phase changes?
Temperature plays a crucial role in phase changes by determining whether a substance exists as a solid, liquid, or gas. By increasing or decreasing the temperature, the energy of the molecules changes, affecting their arrangement and movement. During phase changes, such as melting, freezing, vaporization, condensation, and sublimation, temperature influences the balance between the attractive forces holding molecules together and the kinetic energy of the molecules. Therefore, specific temperatures are associated with each phase change, providing a clear distinction between the different states of matter.
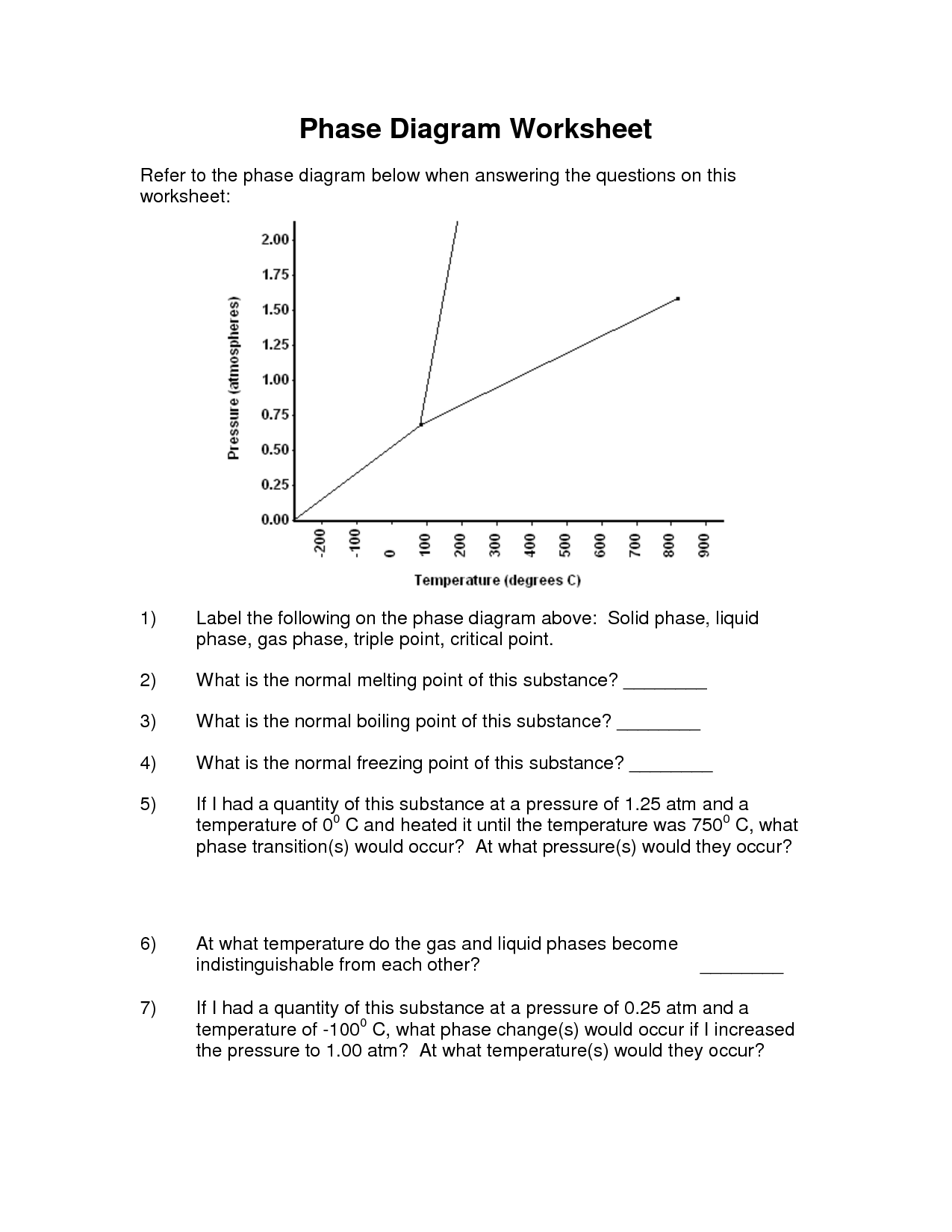
How does pressure affect phase changes?
Pressure can affect phase changes by altering the equilibrium between the different phases of a substance. For most substances, increasing pressure tends to favor the phase with higher density. For example, increasing pressure can cause a solid to melt into a liquid or a liquid to vaporize into a gas. Conversely, decreasing pressure can have the opposite effect, causing a gas to condense into a liquid or a liquid to freeze into a solid. This relationship between pressure and phase changes is described by the phase diagram of a substance.
Explain the concept of latent heat.
Latent heat refers to the amount of heat energy that is absorbed or released during a phase change of a substance without a change in temperature. This energy is used to either break or form intermolecular bonds during the transition between solid, liquid, and gas phases. When a substance changes its state, latent heat is either absorbed (during melting and vaporization) or released (during freezing and condensation) without affecting its temperature. This concept is important in understanding processes like melting ice or boiling water, where the heat energy is used to change the state of a substance rather than raising its temperature.
What are some examples of phase changes in everyday life?
Some examples of phase changes in everyday life include boiling water to make tea, melting butter on toast, freezing water to make ice cubes, condensation forming on a glass of cold water, and steam rising from a pot of simmering soup. These phase changes involve the transitions between solid, liquid, and gas states of matter due to changes in temperature and pressure.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



























Comments