Periodic Table Basics Worksheet
The periodic table is a fundamental tool for understanding the elements of our world. Designed to organize and categorize the building blocks of matter, it provides a visual representation of the countless entities that make up our universe. For educators and students alike, worksheets can serve as an invaluable resource for reinforcing knowledge and exploring the properties of each element. By delving into this periodic table basics worksheet, you can deepen your understanding of the subject and strengthen your grasp of chemistry's fundamental principles.
Table of Images 👆
- Periodic Table Basics Worksheet Answer Key
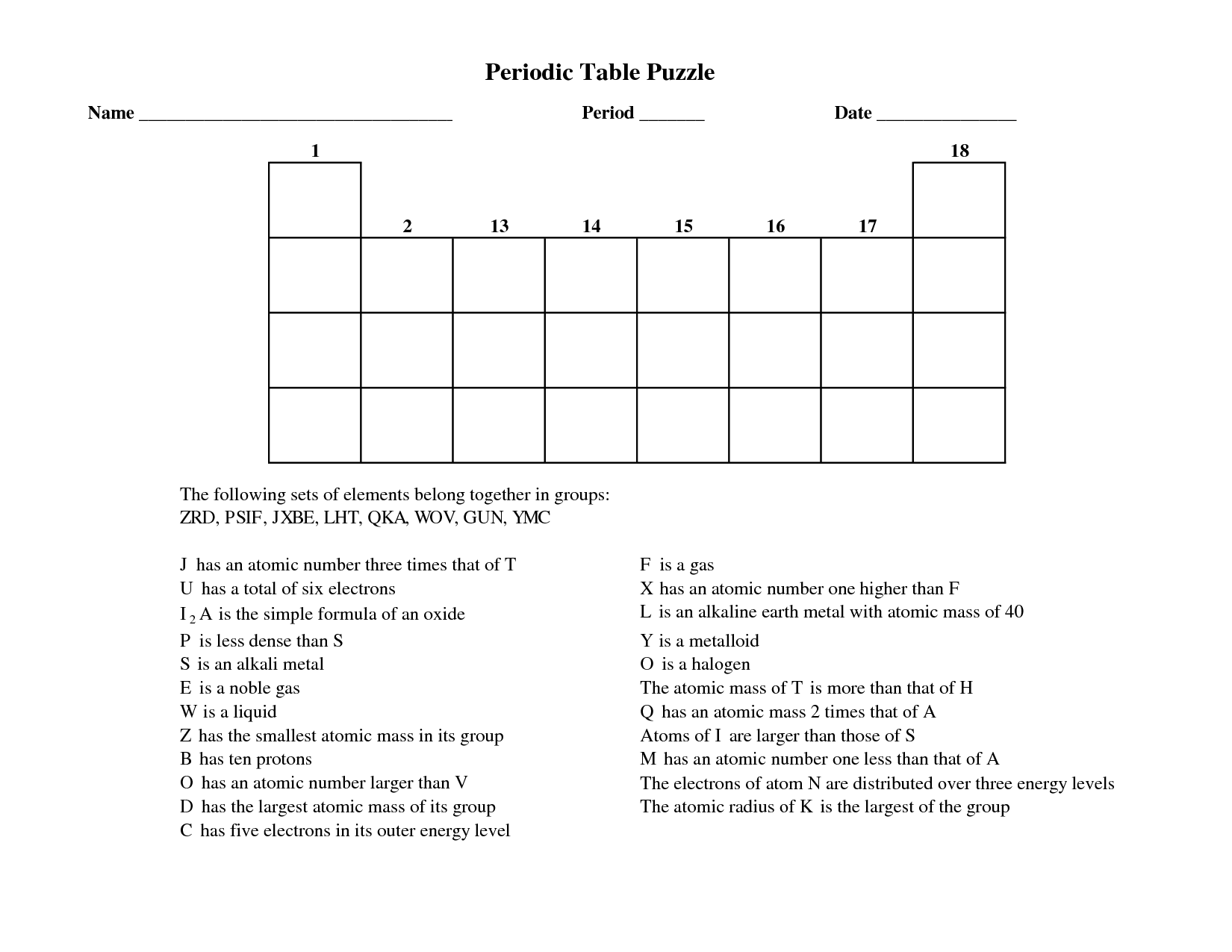
- Periodic Table Puzzle Worksheet Answers
- Chemistry Periodic Table Worksheet Answers
- Periodic Table Worksheet Answers
- Alien Periodic Table Worksheet Answers
- Alien Periodic Table Worksheet
- Balancing Chemical Equations Worksheet Answer Key
- Word Search Answers T. Trimpe 2003 Science Spot
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is the Periodic Table?
The Periodic Table is a tabular arrangement of chemical elements, organized based on their atomic number, electron configuration, and recurring chemical properties. It consists of rows called periods and columns called groups, with elements sharing similar characteristics grouped together. The Periodic Table serves as a valuable tool for scientists to understand the properties and relationships of the different elements, aiding in the study and prediction of chemical reactions and behaviors.
How many elements are currently known?
As of June 2021, there are currently 118 known elements in the periodic table.
What is an element?
An element is a substance that cannot be broken down into simpler substances by chemical means. Each element is made up of atoms that have the same number of protons in their nuclei. Elements are the building blocks of all matter in the universe and are organized in the periodic table based on their atomic number and chemical properties.
How are elements organized in the Periodic Table?
Elements in the Periodic Table are organized based on their atomic number, which is the number of protons in the nucleus of an atom. The table is divided into periods (rows) and groups (columns), with elements arranged in order of increasing atomic number from left to right and top to bottom. Elements within the same group have similar chemical properties due to their similar electron configurations, while elements within the same period have the same number of electron shells. This organization helps to illustrate important trends and relationships among the elements.
What is the atomic number of an element?
The atomic number of an element is the number of protons found in the nucleus of each atom of that element. It is a unique identifier for each element on the periodic table and determines its placement and properties within the table.
What is an atomic symbol?
An atomic symbol is a one or two-letter abbreviation used to represent an element on the periodic table. Each element has a unique atomic symbol, which is derived from the element's name in English, Latin, or another language. This symbol is often used in chemical formulas and equations to represent specific elements.
What is the atomic mass of an element?
The atomic mass of an element is the average mass of an atom of that element, measured in atomic mass units (amu). It takes into account the different isotopes of the element and their relative abundances.
How can you determine the number of protons in an element?
You can determine the number of protons in an element by looking at the atomic number of the element, which is typically found on the periodic table. The atomic number represents the number of protons in the nucleus of an atom of that element.
What is the significance of the periods and groups in the Periodic Table?
The Periodic Table is organized into periods (rows) and groups (columns) to showcase the trends and patterns in the properties of elements. The periods represent the number of energy levels an element's atoms have, while the groups represent elements with similar chemical properties due to the same number of valence electrons. Understanding these patterns helps predict an element's reactivity, the formation of compounds, and its physical and chemical properties based on its position in the table.
How are elements classified as metals, nonmetals, and metalloids?
Elements are classified as metals, nonmetals, and metalloids based on their physical and chemical properties. Metals are typically solid, shiny, malleable, and good conductors of heat and electricity. Nonmetals are generally brittle, dull, and poor conductors of heat and electricity. Metalloids share properties of both metals and nonmetals, displaying some characteristics of each. This classification is determined by the elements' position on the periodic table and their atomic structure, which influences their behavior and reactivity.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.




































Comments