Periodic Table 8th Grade Science Worksheet
The Periodic Table 8th Grade Science Worksheet is a valuable resource designed to help eighth-grade students better understand the arrangement and properties of elements. With clear and concise questions related to the periodic table, this worksheet allows students to engage with the subject matter and reinforce their learning. Whether you're a teacher seeking a supplementary activity or a student looking for extra practice, this worksheet is a helpful tool for enhancing knowledge about the periodic table in an informative and enjoyable way.
Table of Images 👆
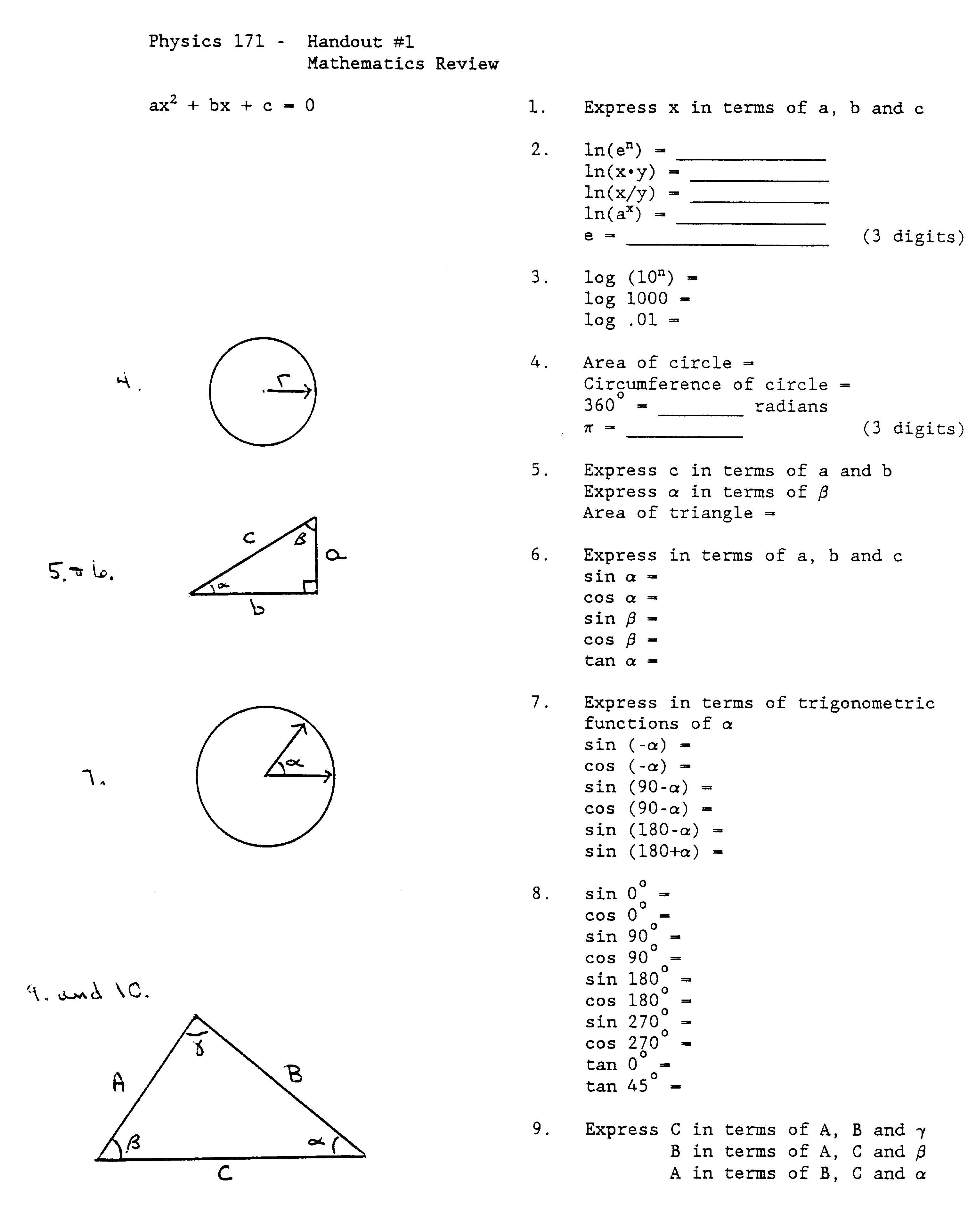
- Physics Math Review Worksheet
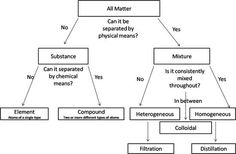
- Matter Classification Flow Chart Worksheet
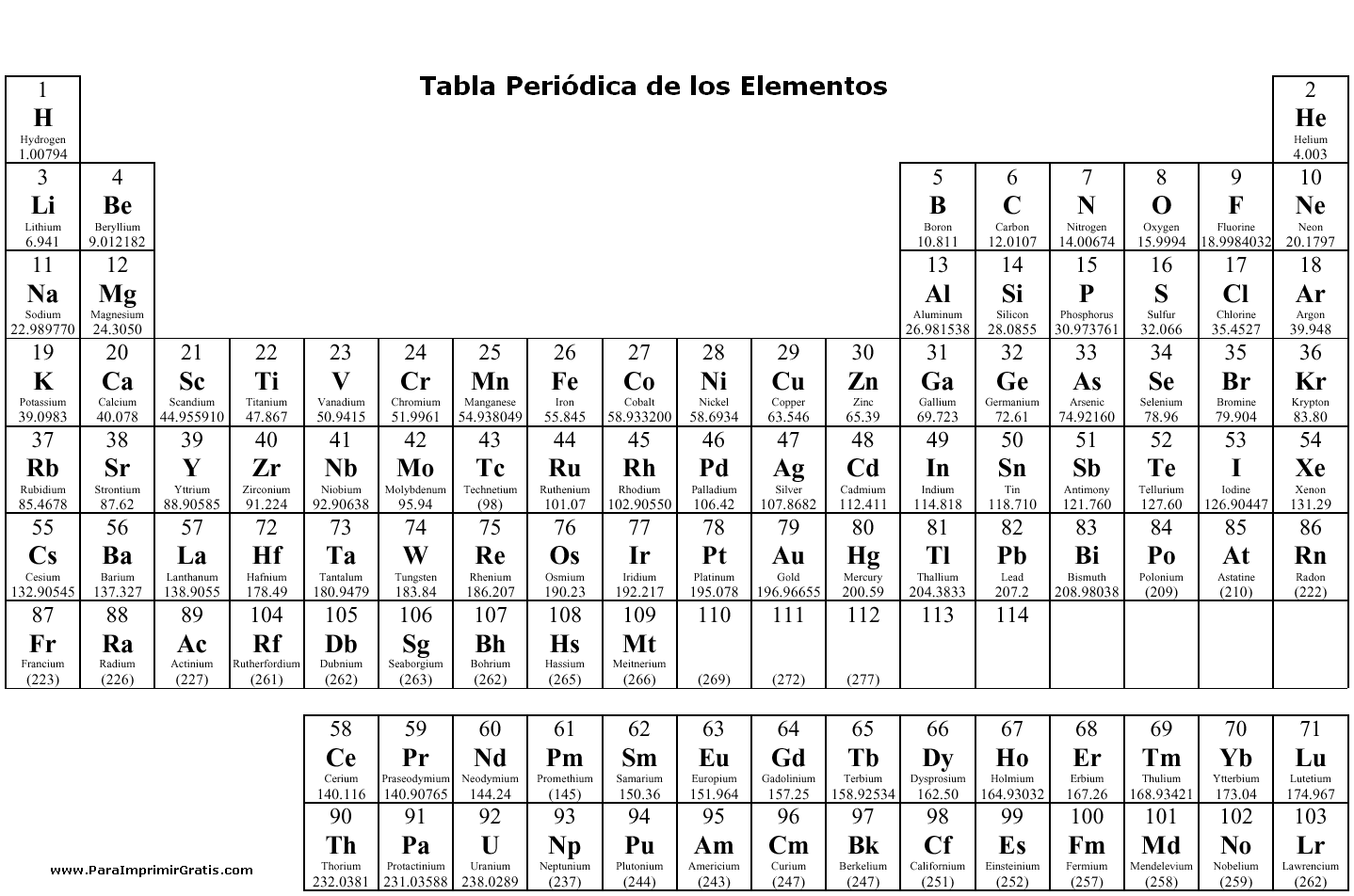
- Periodic Table with Element Charges
- Darwins Theory of Evolution Worksheet
- 6th Grade Math Graph Worksheets
- Lewis Dot Structure Worksheet
- Atoms and Molecules in Chemical Formulas Worksheet
- Balancing Chemical Equations Worksheet Answers
- Vocabulary Words Self Portrait
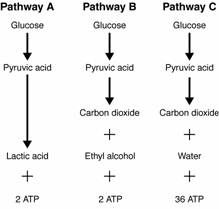
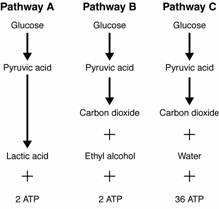
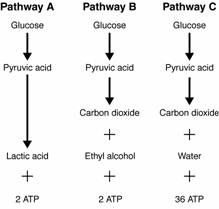
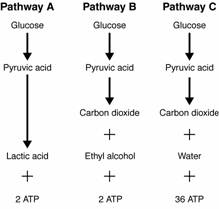
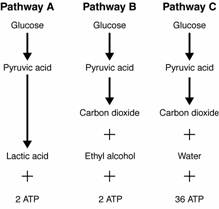
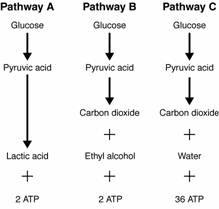
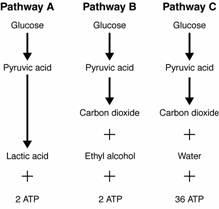
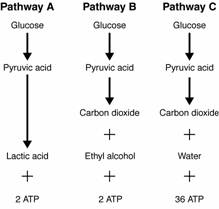
- Sentences Using the Word Glycolysis
More Science Worksheets
6 Grade Science WorksheetsScience Heat Energy Worksheets with Answer
Science Worksheets Light and Sound
7th Grade Science Cells Worksheets
Worksheets Life Science Vocabulary
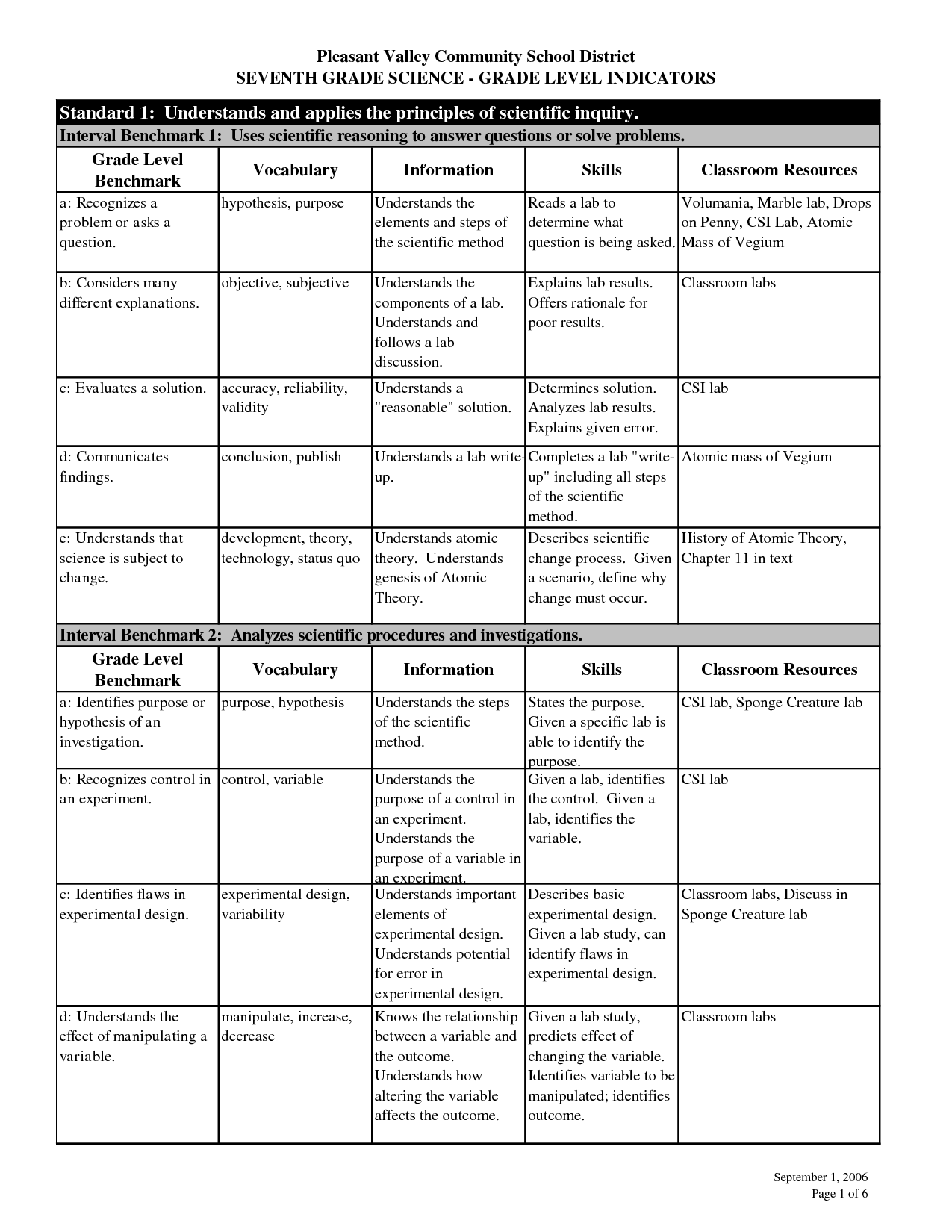
8th Grade Science Scientific Method Worksheet
Science Worksheets All Cells
What is the Periodic Table?
The Periodic Table is a tabular arrangement of elements based on their atomic number, electron configuration, and recurring chemical properties. It organizes all known chemical elements in a concise and systematic manner, grouping elements with similar properties together in rows and columns. The table is an essential tool in chemistry for predicting element behavior, understanding atomic structure, and studying chemical reactions.
Who is credited with creating the Periodic Table?
Russian chemist Dmitri Mendeleev is credited with creating the Periodic Table in 1869. Mendeleev's organization of the elements by atomic mass and predicted the properties of elements that had not yet been discovered based on gaps in his table. His work laid the foundation for the modern understanding of chemical elements and their relationships.
How is the Periodic Table organized?
The Periodic Table is organized by atomic number, which is the number of protons in an atom's nucleus. Elements are arranged in rows called periods and columns called groups or families, with elements in the same group having similar chemical properties. The table is divided into metals, nonmetals, and metalloids, and it is structured so that elements with similar properties are placed in close proximity to each other.
What are periods in the Periodic Table?
Periods in the Periodic Table refer to the horizontal rows that categorize elements based on the number of electron shells they have. Each period represents a new energy level where the outermost electrons reside, starting with the first period at hydrogen and helium with one electron shell and ending with the seventh period at francium and radium with seven electron shells. Elements within the same period often share similar chemical properties due to their electron configurations.
What are groups in the Periodic Table?
Groups in the Periodic Table are vertical columns that contain elements with similar chemical properties. These elements have the same number of valence electrons, which determines their chemical reactivity and behavior. The elements in the same group often share similar physical and chemical characteristics, making it easier to predict their properties based on their position in the table.
How many elements are currently known and listed in the Periodic Table?
There are currently 118 elements known and listed in the Periodic Table.
What information do the atomic number and atomic symbol provide for each element?
The atomic number of an element represents the number of protons in the nucleus of its atom, which determines the element's identity. The atomic symbol, typically a one or two-letter abbreviation, is used to represent the element in the periodic table and chemical formulas. It provides a shorthand way to identify and differentiate elements based on their unique symbols.
How are elements arranged in order of increasing atomic number?
Elements are arranged in order of increasing atomic number in the periodic table. Each element is assigned a unique atomic number based on the number of protons in its nucleus. As you move from left to right in a row or from top to bottom in a column on the periodic table, the atomic number of the elements increases, reflecting the increase in the number of protons in their nuclei. This organized arrangement helps scientists predict the properties and behavior of elements based on their location in the periodic table.
What do elements in the same group have in common?
Elements in the same group have the same number of electrons in their outermost energy level, also known as valence electrons. This results in similar chemical behavior among these elements, such as similar reactivity and bonding properties. Additionally, elements in the same group tend to have similar physical properties and trends in their atomic size and ionization energy.
What are some key properties of metals, nonmetals, and metalloids?
Metals are good conductors of heat and electricity, have a shiny appearance, are malleable and ductile, and tend to lose electrons in chemical reactions. Nonmetals, on the other hand, are poor conductors of heat and electricity, have a dull appearance, are brittle, and tend to gain electrons in chemical reactions. Metalloids possess properties of both metals and nonmetals, such as semi-conducting behavior, varying degrees of conductivity, and some ductility and malleability.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.
























Comments