Naming Binary Ionic Compounds Worksheet
Are you a chemistry student who wants to reinforce your knowledge of naming binary ionic compounds? Look no further! This worksheet is designed to assist you in mastering the skill of naming these compounds by providing clear and concise explanations of the rules and guiding you through various practice problems. Whether you are a beginner or just need some extra practice, this worksheet will be a valuable resource to enhance your understanding of this important topic in chemistry.
Table of Images 👆
- Naming Acids Worksheet Answer Key
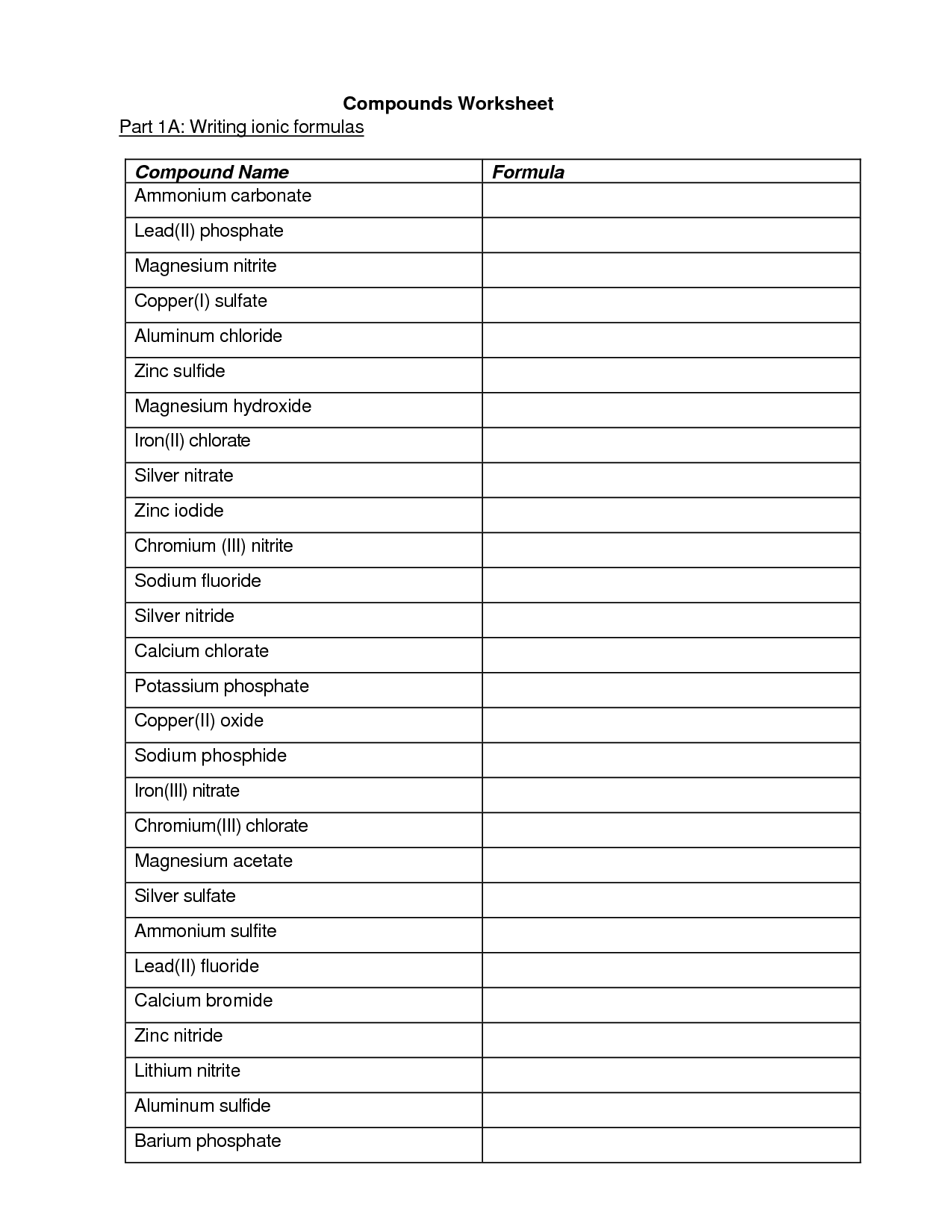
- Writing Ionic Compound Formula Worksheet
- Binary Ionic Compounds Worksheet Answers
- Naming Ionic Compounds Worksheet
- Binary Molecular Compounds Worksheet Answers
- Compounds Polyatomic Ions Ionic
- Rules for Naming Binary Ionic Compounds
- Chemistry Writing Chemical Formulas
- Formulas for Covalent Compounds for Antimony Tribromide
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is a binary ionic compound?
A binary ionic compound is a chemical compound composed of two different elements where one element is a metal and the other is a non-metal. These compounds are formed through the transfer of electrons from the metal to the non-metal, resulting in the formation of positively charged cations and negatively charged anions that are held together by ionic bonds.
How are binary ionic compounds named?
Binary ionic compounds are named by first stating the cation (positive ion) followed by the anion (negative ion). The cation is named using the element's name as is, whereas the anion is named by changing the ending of the element's name to "-ide". For example, sodium chloride is a binary ionic compound made up of the sodium cation and chloride anion.
What is the purpose of the naming system for binary ionic compounds?
The purpose of the naming system for binary ionic compounds is to clearly and universally identify the components involved in the compound by using a systematic naming convention that indicates the type and ratio of elements present. This naming system helps chemists and scientists communicate effectively about the composition of compounds, facilitating better understanding, classification, and organization within the field of chemistry.
What is the role of cations in binary ionic compounds?
Cations play a crucial role in binary ionic compounds by providing the positive charge that balances the negative charge of anions. Cations are formed when atoms lose electrons, leading to a positive charge, while anions gain electrons, resulting in a negative charge. In a binary ionic compound, cations combine with anions through electrostatic forces to form a neutral compound. The presence of cations ensures that the compound remains electrically balanced, with the total positive charge of the cations equaling the total negative charge of the anions.
What is the role of anions in binary ionic compounds?
Anions in binary ionic compounds act as the negatively charged ions that combine with positively charged cations to form a stable compound through ionic bonding. Anions provide the necessary electrons to balance out the positive charge of the cation, ensuring that the compound maintains overall electrical neutrality. The anions play a crucial role in determining the chemical properties and reactivity of the compound, as they contribute to the overall structure and stability of the binary ionic compound.
How are the charges of the cations determined?
The charges of cations are determined by the number of electrons that the cation has lost. Cations are formed when an atom loses one or more electrons, resulting in a positively charged ion. The charge of a cation is thus equal to the number of electrons lost by the original neutral atom.
How are the charges of the anions determined?
The charges of anions are determined by gaining electrons to achieve a stable electron configuration. Anions are negatively charged ions formed by atoms that have gained one or more electrons. The charge of an anion is equal to the number of electrons gained, which results in the atom having a full outer electron shell, similar to the nearest noble gas configuration, making the anion more stable.
What is the significance of using Roman numerals in the names of some binary ionic compounds?
Using Roman numerals in the names of some binary ionic compounds is significant because it indicates the charge of the transition metal cation in the compound. Since transition metals can have variable charges, the Roman numeral helps distinguish which specific charge the transition metal has in the compound, which is essential for correctly naming and writing the chemical formula of the compound.
How do you determine the formula of a binary ionic compound given its name?
To determine the formula of a binary ionic compound given its name, you need to identify the charges of the ions involved. The charge of the cation (metal) is the same as its oxidation number, while the charge of the anion (nonmetal) is determined based on its position in the periodic table. Once you know the charges, you can balance them to achieve a neutral compound by using subscripts to indicate the number of each ion needed to balance the charges. The final formula is written with the cation listed first, followed by the appropriate subscripts for each ion.
Can you provide an example of a binary ionic compound and explain how to name it?
Sure! An example of a binary ionic compound is sodium chloride (NaCl). To name it, you would simply write the name of the cation (sodium) followed by the name of the anion (chloride), without any prefixes or Roman numerals. This is a general rule for naming binary ionic compounds, where the cation is always named first followed by the anion.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.




























Comments