Molecular and Ionic Equations Worksheet
Are you struggling to grasp the concepts of molecular and ionic equations? If so, you're not alone. Many students find these topics to be challenging and may need additional practice to truly understand them. Luckily, there is a valuable resource available to help you on your journey - the Molecular and Ionic Equations Worksheet. This worksheet is designed specifically for students who are looking to reinforce their understanding of these equations and improve their problem-solving skills in chemistry.
Table of Images 👆
- Common Ionic Compounds List
- Chemfiesta Balancing Equations Practice Worksheet Answers
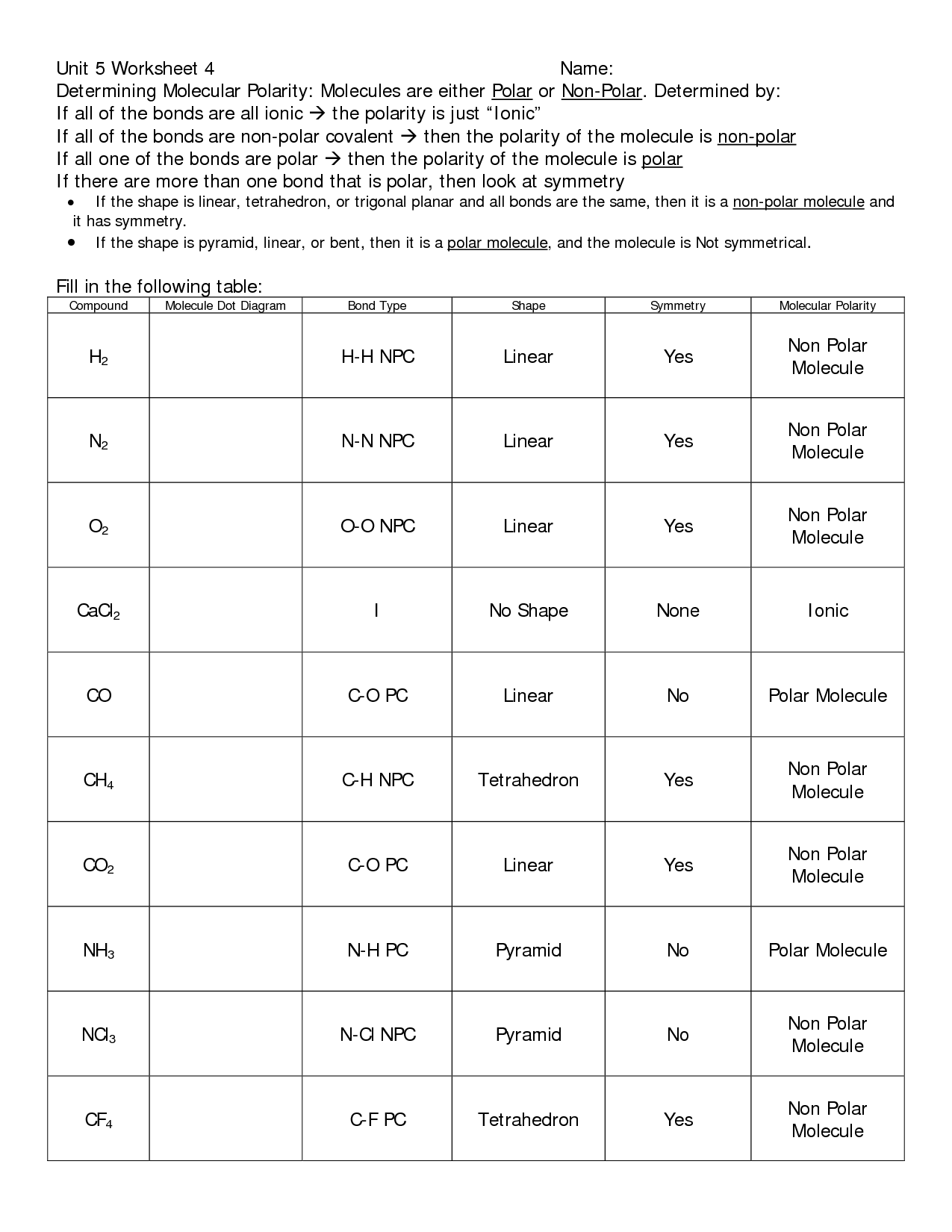
- Naming Covalent Compounds Worksheet Key
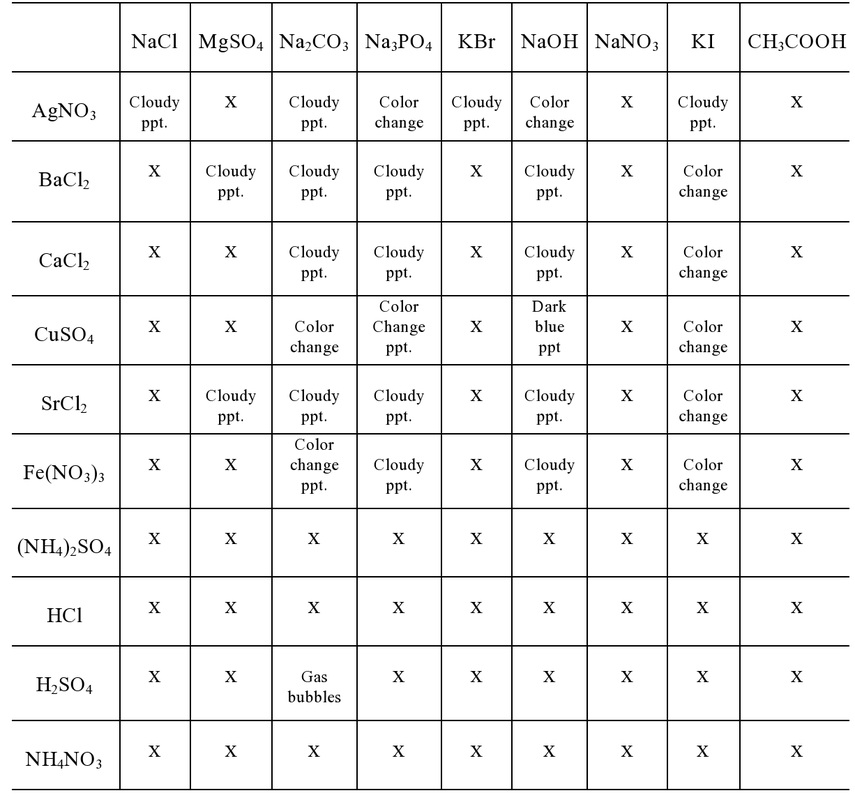
- Ionic Reactions Lab DataTable
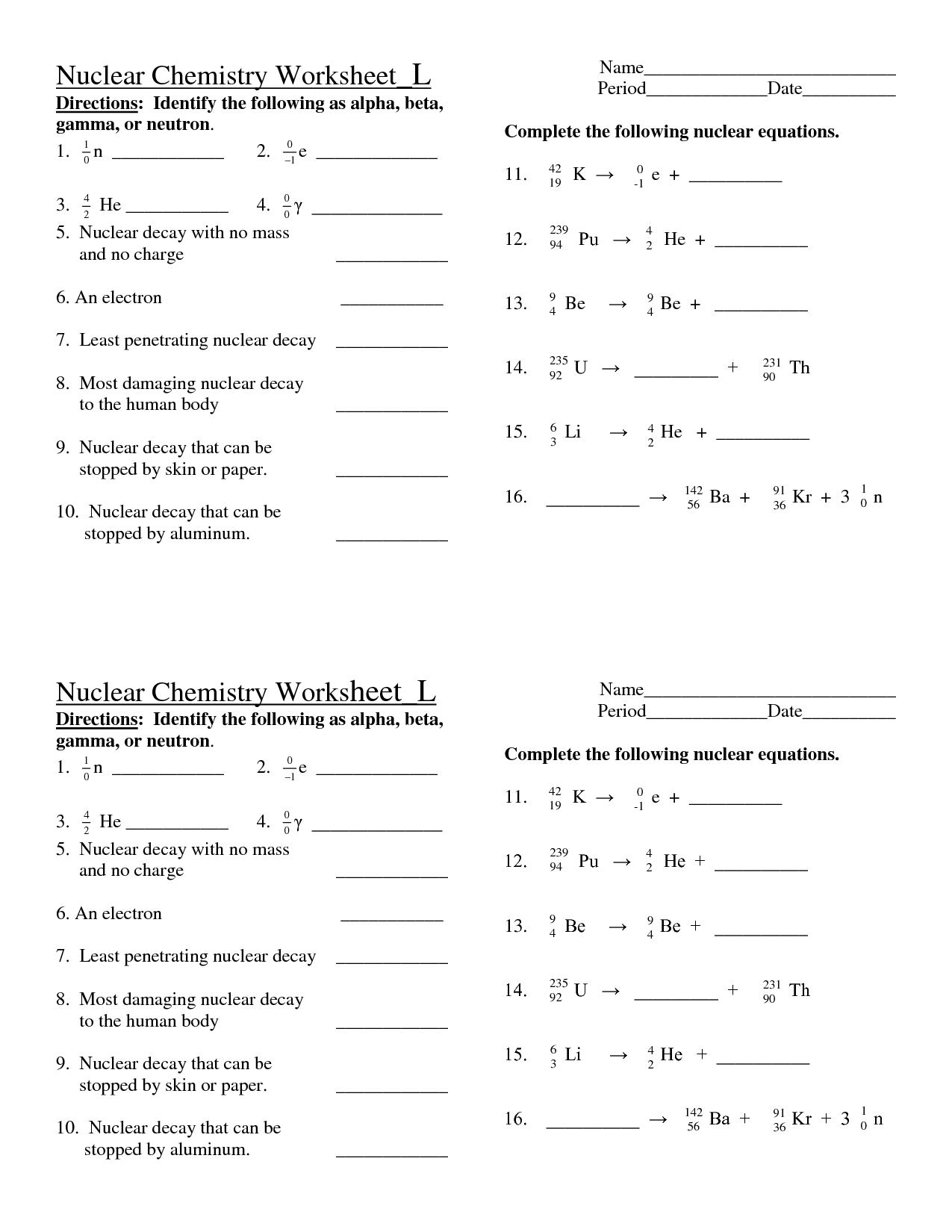
- Chemistry Nuclear Decay Worksheet Answers
- Chemical Nomenclature Worksheet
- Net Ionic Equations Worksheet
- Ionic Bonding Worksheet Answer Key
- Binary Ionic Compounds Formulas Worksheet
- Chemical Formula Copper 2 Phosphate
- Net Ionic Equation Worksheet Answers
- Chemical Formula Definition Chemistry
- Chapter 12 Stoichiometry Answer Key PDF
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
Define a molecular equation.
A molecular equation is a representation of a chemical reaction using the chemical formulas of all reactants and products, without indicating the ionic species involved. It provides a simplified overview of the chemical reaction, showing the substances involved and their ratios, but does not show the dissociation of ionic compounds into their constituent ions, which is typically seen in ionic equations.
What is an ionic equation?
An ionic equation is a chemical equation that shows only the ions that participate in a chemical reaction, omitting the spectator ions. It highlights the species that undergo a change in oxidation state or are directly involved in the reaction, which helps in understanding the essence of the reaction without unnecessary details.
How are molecular equations and ionic equations different?
Molecular equations show all reactants and products as complete compounds, while ionic equations focus on the dissociation of compounds into their constituent ions in aqueous solutions. Molecular equations do not differentiate between ionic and molecular compounds, whereas ionic equations only display the species participating in the chemical reaction as ions.
What is the purpose of balancing a chemical equation?
The purpose of balancing a chemical equation is to ensure that the law of conservation of mass is obeyed. Balancing the equation involves adjusting the number of atoms of each element on both the reactant and product sides to ensure that the quantity of each element remains the same before and after the reaction. This helps to accurately represent the reaction that is occurring and allows for the calculation of the quantities of reactants and products involved in the chemical reaction.
Explain why empirical formulas are used in molecular equations.
Empirical formulas are used in molecular equations because they represent the simplest whole-number ratio of atoms in a compound. This simplifies calculations and allows for a better understanding of the composition of a substance. By focusing on the empirical formula, chemists can analyze chemical reactions, predict the products formed, and determine the stoichiometry of the reaction without getting bogged down in the exact number of atoms of each element in a molecule. Additionally, empirical formulas provide a consistent way to communicate the basic composition of compounds in a clear and concise manner.
Describe the process of writing a balanced molecular equation.
To write a balanced molecular equation, start by writing the chemical formulas of the reactants and products involved in the chemical reaction. Next, balance the equation by adjusting the coefficients in front of the chemical formulas to ensure that the number of atoms of each element is the same on both sides of the equation. Use the principle of conservation of mass to guide you in balancing the equation. Finally, double-check your work to ensure that the equation is balanced, meaning that the total number of atoms of each element is the same on both sides of the reaction arrow.
How would you convert a molecular equation into an ionic equation?
To convert a molecular equation into an ionic equation, first write out the chemical equation as it is with all reactants and products. Next, identify any strong electrolytes in the equation and break them down into their respective ions. Do not break down any weak electrolytes or non-electrolytes. Finally, rewrite the equation with the strong electrolytes as separate ions while keeping the weak electrolytes and non-electrolytes in molecular form. This results in the ionic equation which represents the dissociation of ions in the solution.
What are spectator ions in an ionic equation?
Spectator ions in an ionic equation are ions that do not participate in the chemical reaction and remain unchanged before and after the reaction. They are present in the solution but do not contribute to the formation of products or affect the overall reaction. Spectator ions can be omitted from the equation to simplify it and focus on the ions that actually participate in the reaction.
Why are spectator ions important in chemical reactions?
Spectator ions are important in chemical reactions because they do not participate in the actual reaction but help maintain charge neutrality and balance the overall equation. By keeping the same amount of positive and negative charges on both sides of the reaction, spectator ions ensure that the reaction follows the law of conservation of mass and charge. Identifying and including spectator ions in reactions is crucial for accurately writing and balancing chemical equations.
What can be inferred from a balanced ionic equation?
From a balanced ionic equation, it can be inferred that the number of each type of atom present and the total charge are conserved on both sides of the equation. This indicates that the reaction obeys the law of conservation of mass and charge, showing that no atoms are created or destroyed during the chemical reaction.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.












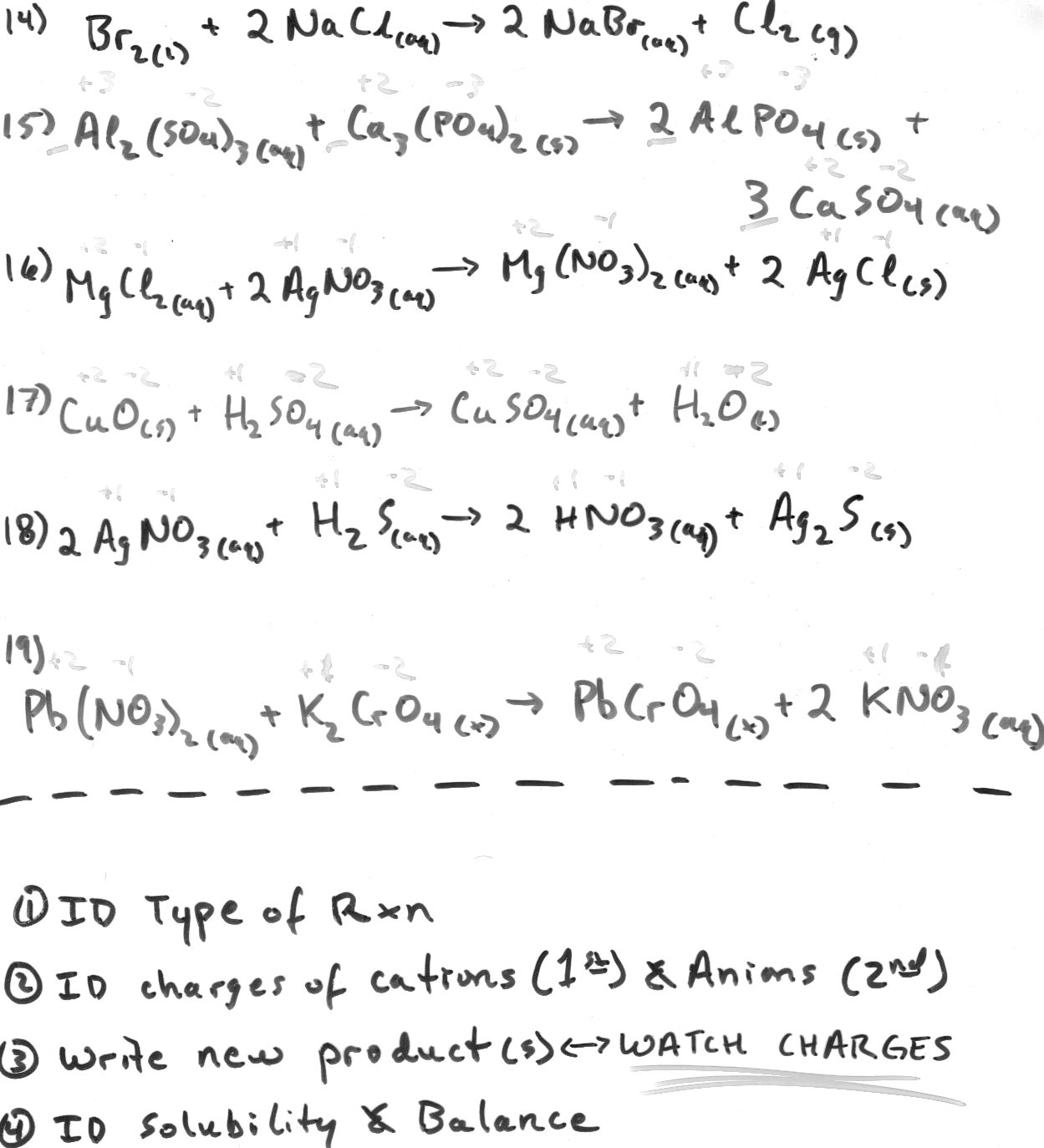
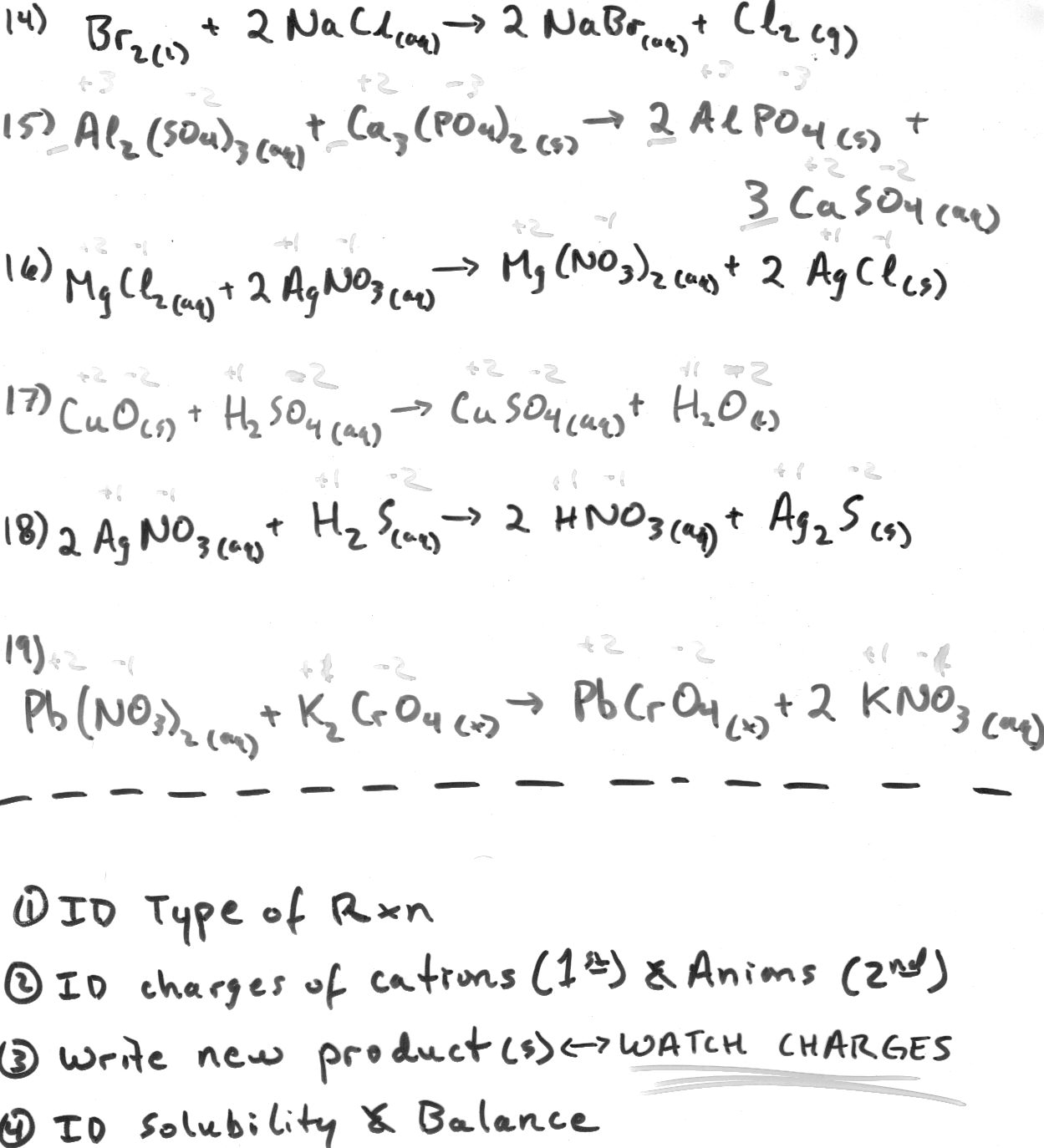
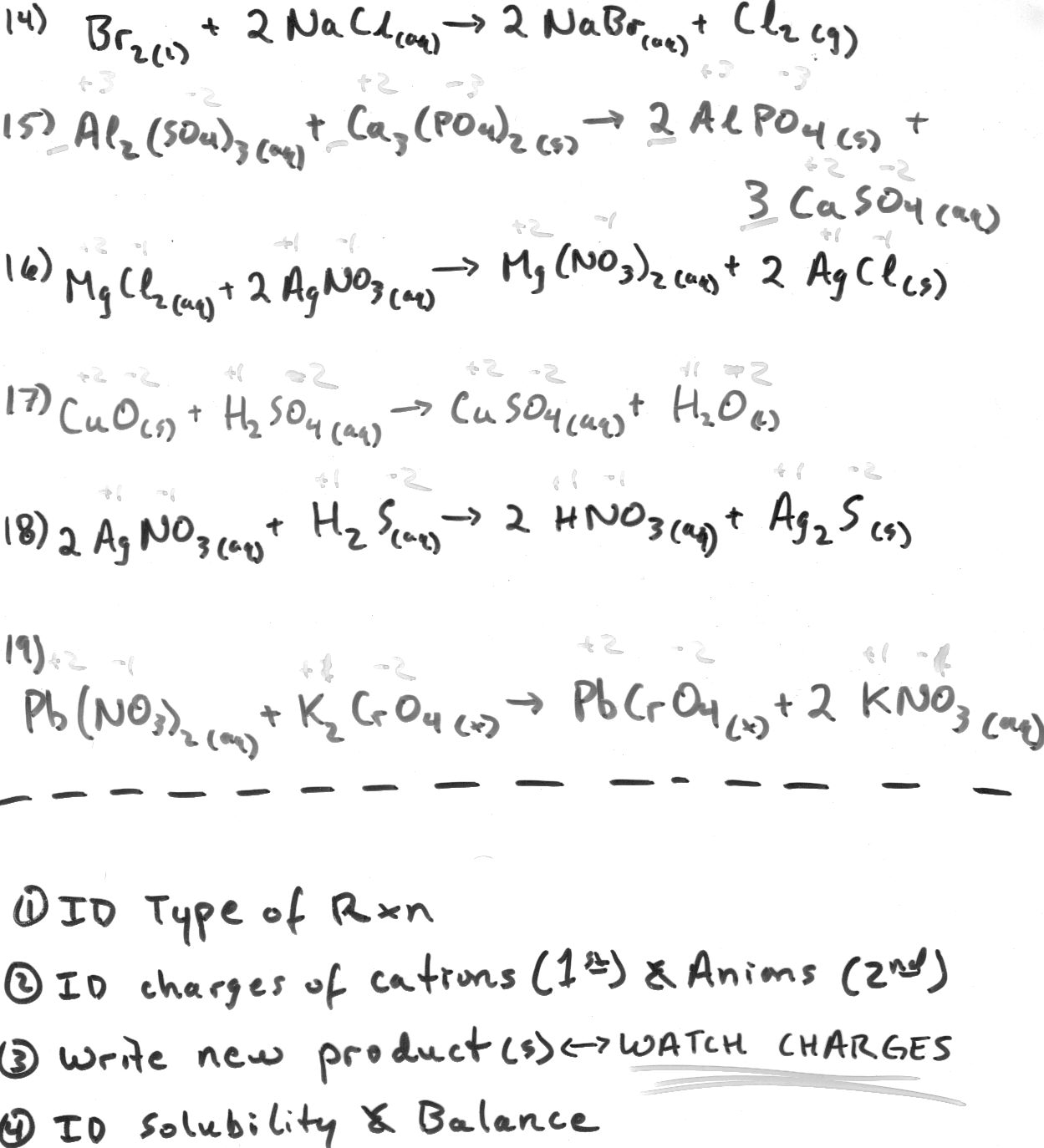















Comments