Molecular and Empirical Formula Worksheet Answers
Are you a chemistry student searching for a reliable resource to practice molecular and empirical formula calculations? Look no further! Our comprehensive Molecular and Empirical Formula Worksheet Answers are here to support your learning journey. Designed for both high school and college-level chemists, this worksheet provides a range of practice problems to reinforce your understanding of molecular and empirical formulas.
Table of Images 👆
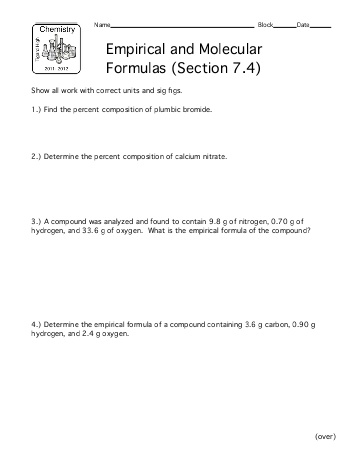
- Molecular and Empirical Formula Worksheet
- Molecular and Empirical Formula Worksheet Answer Key
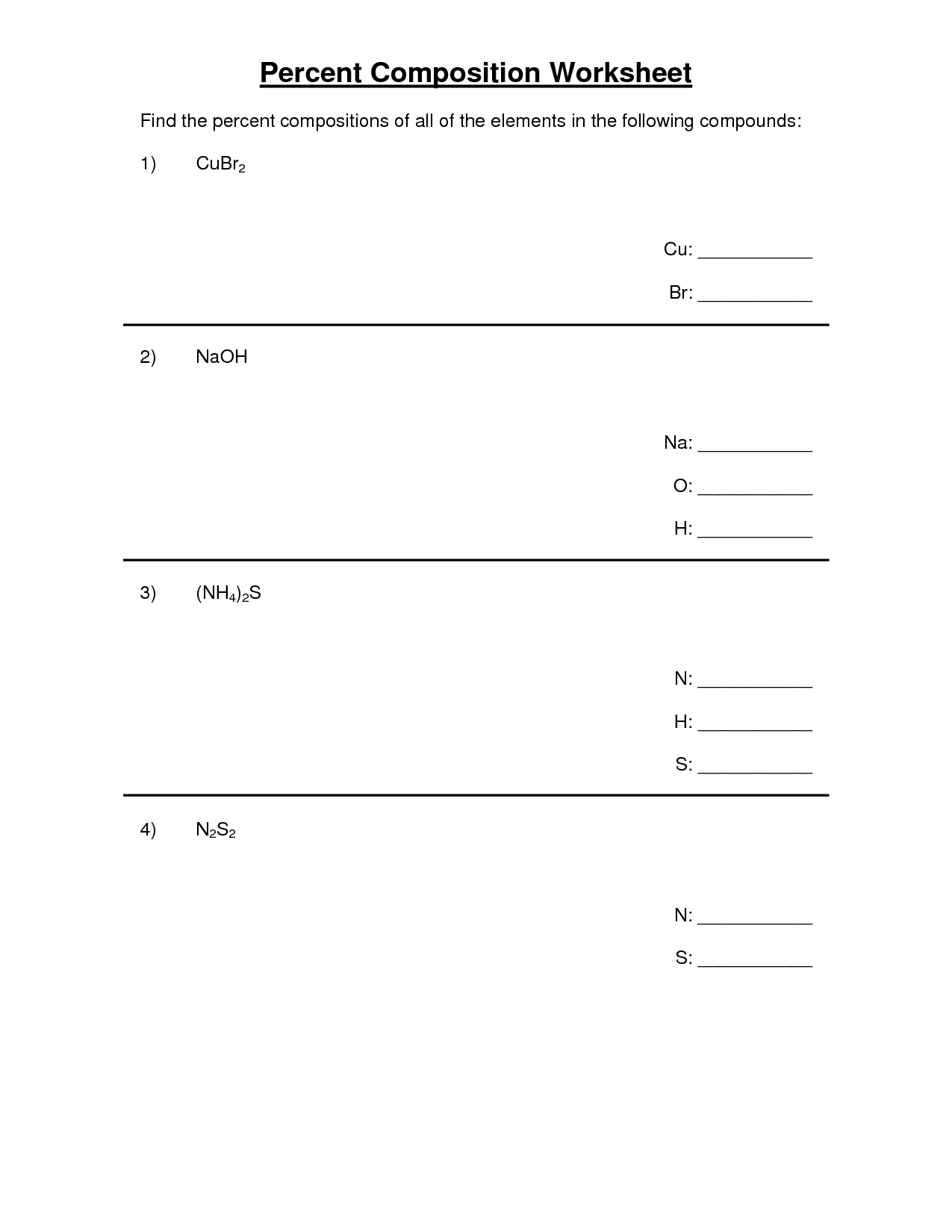
- Percent Composition Empirical Formula Worksheet Answers
- Percent Composition Worksheet Answer Key
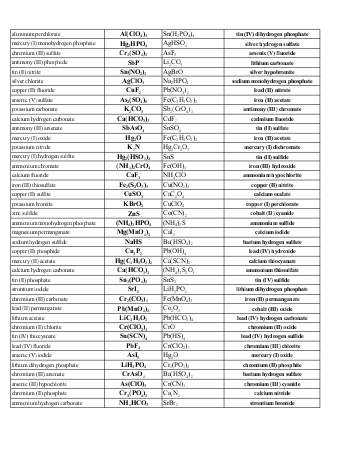
- Naming Ionic Compounds Worksheet Answer Key
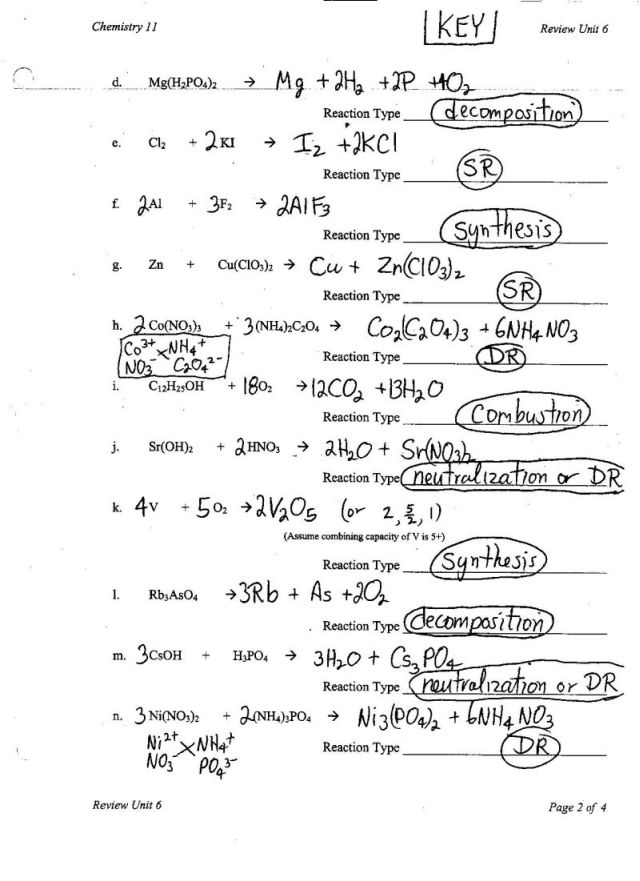
- Types of Chemical Reactions Worksheet Answer Key
- Naming Compounds and Writing Formulas Answers
- Composition of Blood and Functions Worksheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
All Amendment Worksheet
Symmetry Art Worksheets
Daily Meal Planning Worksheet
What is a molecular formula?
A molecular formula is a representation of a molecule that shows the types and numbers of atoms present in the molecule. It provides information about the atomic composition of the compound and is a concise way to denote the chemical makeup of a substance by indicating the elements present and the exact number of each type of atom in the molecule.
How is a molecular formula different from an empirical formula?
A molecular formula represents the actual number of atoms of each element in a molecule, while an empirical formula represents the simplest whole-number ratio of atoms of each element in a compound. Molecular formulas provide the exact composition of a molecule, while empirical formulas offer a simplified, more general representation of the compound's elemental makeup.
What information does a molecular formula provide about a compound?
A molecular formula provides the exact number and types of atoms present in a molecule of a compound. It gives the ratio of each element in the compound and can help determine the compound's molar mass and structure, allowing for identification and study of its chemical properties.
How is a molecular formula determined experimentally?
To determine a molecular formula experimentally, scientists typically use analytical techniques such as mass spectrometry and elemental analysis. Mass spectrometry helps determine the molecular weight of a compound based on the mass-to-charge ratio of its ions, providing information on the number of atoms present in the molecule. Elemental analysis involves determining the percentages of different elements present in the compound, which allows for the calculation of the empirical formula. By combining the information from these techniques, scientists can deduce the molecular formula of a compound by determining the actual number and types of atoms present in the molecule.
What is an empirical formula?
An empirical formula is the simplest whole number ratio of atoms of each element in a compound. It gives the relative number of atoms of each element present in a compound but does not provide the actual number of atoms in a molecule. This formula is often used in chemistry to represent the composition of a compound in a simplified form.
How is an empirical formula determined?
An empirical formula is determined based on the simplest whole-number ratio of atoms present in a compound, obtained from the elemental composition of the compound. This is typically determined through experimental data such as mass percentage or spectral analysis, where the number of moles of each element in the compound is determined and then divided by the smallest mole value to obtain whole-number ratios. The resulting ratios give the empirical formula of the compound.
Can a compound have multiple empirical formulas?
No, a compound will have only one empirical formula, which represents the simplest whole number ratio of the elements in the compound. While a compound may have multiple molecular formulas, depending on its molecular structure, it will always have a unique empirical formula that is derived from the relative proportions of the elements present.
How can the empirical formula be used to determine the molecular formula?
The empirical formula provides the simplest whole number ratio of atoms in a compound. To determine the molecular formula from the empirical formula, the molar mass of the empirical formula must be compared to the experimentally determined molar mass of the compound. By dividing the experimental molar mass by the molar mass of the empirical formula, you can find the factor by which the empirical formula must be multiplied to get the molecular formula. This factor is then applied to the subscripts in the empirical formula to determine the molecular formula.
What is the relationship between the molecular formula and the empirical formula?
The molecular formula shows the exact number of each type of atom in a molecule, while the empirical formula shows the simplest whole-number ratio of atoms in a compound. The molecular formula can be a multiple of the empirical formula if the compound contains multiple units of the empirical formula.
How can the molecular and empirical formulas help in understanding the composition of a compound?
The molecular formula provides the exact number and type of atoms present in a compound, while the empirical formula gives the simplest whole-number ratio of atoms. By comparing the molecular and empirical formulas, one can gain insight into the actual arrangement of atoms within the compound. This information helps in understanding the composition of the compound, determining its molecular structure, and predicting its properties and behavior.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.






















Comments